Irrigating the root canals with antimicrobial solutions helps to decrease or completely eliminate microorganisms from the root canal system. Incomplete elimination of microorganisms from the root canals may lead to the persistence or survival of the microorganisms in the complex root canal system leading to the failure of endodontic treatment [1]
During the endodontic treatment different irrigating solutions are used for the disinfection of root canal system. The disadvantages of some of the irrigating solutions include limited antimicrobial activity, inability to penetrate into root canal dentin, non selectivity for host cells, toxicity and allergic to periapical tissues [4]. Till-date no ideal irrigating solution is available. There are studies which compared the antimicrobial efficacy of chlorhexidine, sodium hypochlorite but there has been no published study to date which compared the antimicrobial efficacy of sparfloxacin and augmentin against E.faecalis as root canal irrigating solutions.
Sparfloxacin is an antimicrobial agent which belongs to the piperazinyl quinolone class. It acts by inhibiting DNA gyrase, a bacterial topoisomerase IV. DNA gyrase assists in DNA replication, repair, deactivation and transcription [5]. Co-amoxiclav (Trade name: Augmentin) is an oral antibiotic. It is a combination of the semisynthetic antibiotic Amoxicillin and the lactamase inhibitor, Clavulanate potassium (the potassium salt of clavulanic acid). Clavulanic acid blocks the active sites of β-lactamase enzymes. Clavulanic acid protects amoxicillin from degradation by β-lactamase enzymes. Clavulanic acid effectively extends the antibiotic spectrum of amoxicillin. Thus, Co-amoxiclav possesses the properties of a broad-spectrum antibacterial activity in particular bactericidal activity against most gram-positive and gram-negative microorganisms [6].
So, the purpose of this study was to compare the antimicrobial efficacy of two antibiotics 5mcg Sparfloxacin and 30mcg augmentin as experimental root canal irrigating solutions with 2% Chlorhexidine (CHX), 3% sodium hypochlorite (NaOCl), 5% NaOCl against E.faecalis.
Materials and Methods
The present in-vitro study was conducted at Department of Conservative Dentistry and Endodontics and Department of Microbiology, Sri Sai College of Dental Surgery, Vikarabad, Telangana in 2014. Fifteen agar plates were prepared by using Brain Heart Infusion (BHI) agar (Himedia Laboratories, Mumbai, India) in 90mm diameter petri dishes. These petri dishes were stored at room temperature for two days before use to verify that they had remained sterile. A suspension of pure culture of E.faecalis-American Type Culture Collection (ATCC) 29212 (Himedia Laboratories, Mumbai, India) was prepared by adding 1mL of pure culture of E.faecalis to freshly prepared BHI broth. After 24hrs incubation of E.faecalis in BHI Broth, bacterial growth changes were seen in turbidity. Against a ruled paper, it was compared with 0.5 McFarland standard, which was comparable to a bacterial suspension of 1.5x108 Colony Forming Units (CFU)/mL. 0.5 McFarland standard was used as a reference to adjust the turbidity of bacterial suspensions so that the number of bacteria will be within a given range.
The antibacterial sensitivity was performed by agar disc diffusion method. Fifteen BHI agar plates were inoculated by BHI Broth with sterile cotton swabs to provide an even lawn of cells. 3-5 minutes of time was allowed for surface of agar to dry before applying the discs. Sterile paper discs of 6mm in diameter were prepared and autoclaved at a temperature of 121°C for 15min at 15lb pressure. Using a sterile pipette, each 6mm paper test disc was saturated with 10 microliters (μL) of 2% chlorhexidine (CHX) (Vishal Dentocare Private Limited, Ahmedabad, India), 3% sodium hypochlorite (NaOCl) (Vishal Dentocare Private Limited, Ahmedabad, India), 5% NaOCl (Vishal Dentocare Private Limited, Ahmedabad, India) solutions respectively and 0.9% Sodium Chloride solution (NaCl) was used to saturate the control disc.
After inoculation, four saturated paper discs were placed on each agar plate. Three of the discs were saturated with one of the three test solutions and the last paper disc served as a control and was saturated with 0.9% NaCl solution. Standard 5 mcg Sparfloxacin discs (Himedia Laboratories, Mumbai, India), 30mcg Augmentin discs (Himedia Laboratories, Mumbai, India) were placed on separate Brain Heart Infusion agar plates. After placement, the discs were pressed on the surface of the medium to provide uniform contact. BHI agar plates were marked on the bottom of the plate to identify the irrigating solution. Fifteen BHI agar plates were placed in an incubator at 37°C for 48 hrs. After 48hrs, microbial zones of inhibition were measured across the diameter in millimeters (mm) with a pair of Vernier Calipers and the largest diameter was recorded [Table/Fig-1,2 and 3].
Zones of inhibition for 2% CHX, 3% NaOCl, 5% NaOCl and 0.9% NaCl.
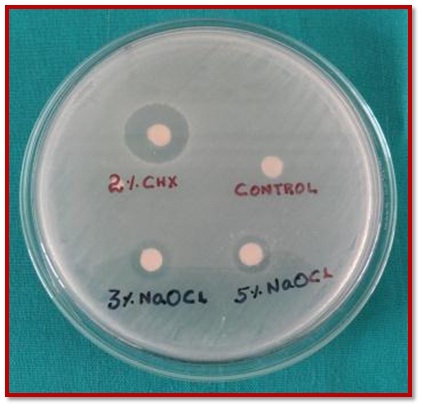
Zone of inhibition for 5mcg Sparfloxacin.

Zone of inhibition for 30mcg Augmentin.

Statistical Analysis
The Statistical Package SPSS (Statistical Package for Social Science, version 4) was used for the statistical analysis. Mean and standard deviation were estimated for all the five different irrigating solutions. The mean values were compared by one-way Analysis of Variance (ANOVA) then appropriately followed by post-hoc tukey test. Post-hoc tukey test was employed to identify the significant irrigating solution. In the present study, the level of significance was set at p = 0.05.
Results
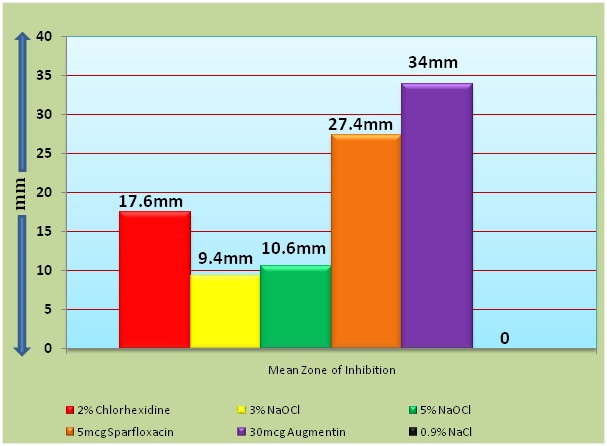
[Table/Fig-4] shows the mean counts, standard deviation and post-hoc tukey test for zones of inhibition between five different irrigating solutions. Mean zones of inhibition was compared for all the five different irrigating solutions and there was statistically significant difference among the groups (p<0.001). [Table/Fig-5] shows the mean zone of inhibitions ranging between 0mm and 34mm. Augmentin 30mcg showed larger zone of microbial Decrease the space between 2% CHX and 3% NaOCl, 5% NaOCl solutions. The zones of inhibition for 3% NaOCl and 5% NaOCl were not significantly different from each other but they both resulted in larger zones of inhibition than control (p<0.05, ANOVA). The control group i.e., 0.9% NaCl solution showed no microbial inhibition.
Mean counts, Standard deviation and Post-hoc tukey test for Zones of Inhibition.
| Irrigating Solutions | N | Mean | SD | p-value | Post-hoc tukey test |
|---|
| 2% CHX (1) | 5 | 17.60 | 1.34 | <0.001 | 1 > 2, 3, 6 |
| 3% NaOCl (2) | 5 | 9.40 | 0.55 | <0.001 | 2 > 6 |
| 5% NaOCl (3) | 5 | 10.60 | 0.55 | <0.001 | 3 > 6 |
| 5mcg Sparfloxacin (4) | 5 | 27.40 | 0.55 | <0.001 | 4 > 1, 2, 3, 6 |
| 30mcg Augmentin (5) | 5 | 34.00 | 0.71 | <0.001 | 5 > 1, 2, 3, 4, 6 |
| 0.9% NaCl (6) | 5 | 0.00 | 0.00 | <0.001 | - |
Graphical representation of Mean Zones of Inhibition of 2% CHX, 3% NaOCl, 5% NaOCl, 5mcg Sparfloxacin, 30mcg Augmentin.
Discussion
In Endodontics, over the years to increase the antimicrobial effect of cleaning and shaping, root canal irrigants are used for elimination of microbes from the root canal. Irrigation plays a vital role in successful endodontic treatment and it is paramount to determine periapical tissue healing. Root canal irrigants should have a broad spectrum of antimicrobial activity to flush out debris from root canal, nontoxic, biocompatible, sterilize the root canal, and dissolve the smear layer [7]. [Table/Fig-6] presents the available root canal irrigants, their advantages, disadvantages and gives some recommendations for their clinical use [8,9].
| Root Canal Irrigants | Concentrations Used | Advantages | Disadvantages |
|---|
| Normal Saline (NaCl) | 0.9% W/V | • Biocompatible• No adverse reactions seen in cases of periapical extrusion | • Don’t have dissolution & disinfecting properties• Don’t possess Antimicrobial (AM) activity• Doesn’t remove smear layer from root canals |
| Sodium Hypochlorite (NaOCl) | 0.5% to 6% | • Has effective AM property• Causes dissolution of pulp & necrotic tissue• Dissolves organic portion of dentin for deeper penetration of intracanal medicaments | • Toxic• In cases of periapical extrusion, results in severe cellular damage & irritant to tissues• Unpleasant odor• Corrosive to instruments |
| Hydrogen Peroxide (H2O2) | 3% | • Has AM property• Effective disinfectant | • Advisable to use NaOCl after H2O2 irrigation, as nascent oxygen from H2O2 causes pressure build up & pain on closing tooth |
| Urea Peroxide | | • Good lubricant• Effective disinfectant | • Dissociates more slowly when compared to H2O2 |
| Iodine Potassium Iodide | 2% to 5% | • Is relatively less toxic in experiments using tissue cultures | • In some patients, allergic reactions are reported |
| Chlorhexidine Digluconate (CHX) | 0.2%,2% | • Has substantive AM activity• More effective on gram-positive bacteria | • Unable to dissolve necrotic tissue remnants• Doesn’t remove biofilm • Less effective on gram-negative bacteria |
| Ethylene diaminetetracetic acid (EDTA) | 17% | • Demineralizes inter tubular dentin• Effectively removes the smear layer | • Prolonged exposure may weaken the root dentin |
| Hydroxyethylidene Bisphosphonate (HEBP) | 18% | • As chelating agent it shows no short-term reactivity with NaOCl | • Prolonged exposure may weaken root dentin |
| Maleic Acid | 5% & 7% | • Effectively removes smear layer from apical 3rd of root canals | • Further evaluation is required |
| Mixture of Tetracycline, an Acid And a Detergent (MTAD) | It is a mixture of 3% Doxycycline 4.25% Citric acid & Detergent (Tween 80,0.5%) | • Has good tissue-dissolving action• Effective in smear layer removal | • Stains root canal dentin• Possible resistance to antibiotic |
| Tetraclean | | • Has low surface tension• Effective against both strictly anaerobic & facultative anaerobic bacteria | • Stains root canal dentin• Possible resistance to antibiotic |
| Ozonated Water | | • Rapid AM activity• Lack of mutagenicity | • If incorrectly used may cause serious medical complications |
| Electrochemically Activated Solution | | • Nontoxic to biological tissues• Effective AM against wide range of microbes | • Further research is required |
| QMix | | • Causes less demineralization of dentin when compared to EDTA• Doesn’t cause erosion of dentin | • Expensive• Causes allergic reactions in some patients |
| Bioglass | | • Has AM activity against wide range of microbes | • More research is needed to evaluate its value in root canal disinfection |
Several natural products like arctium lappa, neem, turmeric, triphala, green tea polyphenols, liquorice, noni, manuka honey, endopam, propolis, cranberrie have been tested as root canal irrigants and they showed better Antimicrobial (AM) efficacy against E.faecalis. Some of the advantages of natural products are their easy availability, less side effects & lack of microbial resistance [10,11].
In dentistry, antibiotics are utilized both systemically and topically. Abbott et al., reported that chronic alveolar infections are seen in pulpless teeth and lesions where blood supply doesn’t reach the pulp. In such cases, by the systemic administration of antibiotics, negligible concentrations reach the root canal. Systemic administration of antibiotics relies on patient’s compliance and their concentration reaching the infected site. To decrease systemic consequences and complications and to increase the efficiency, local administration of antibiotics can be used as root canal irrigating solutions [12]. In the present study two antibiotics i.e., Sparfloxacin and Augmentin were chosen to be used as root canal irrigating solutions and their antimicrobial efficacy was compared with 2% chlorhexidine (CHX), 3% sodium hypochlorite (NaOCl), 5% NaOCl against E.faecalis.
E.faecalis was selected as the test organism in this study because it is the most commonly isolated intracanal bacteria from treatment failure cases, it’s association with persistent apical inflammation and its resistance to elimination by irrigating solutions and medicaments [13].
Presence of E.faecalis in the root canals during root canal filling lowers the rate of endodontic treatment success. According to Hancock et al., E.faecalis is found in filled root canals regardless of the antimicrobials that were used during treatment [14]. Many studies reported the susceptibility of E.faecalis to various antibiotics. Some studies reported that E.faecalis is highly susceptible to amoxicillin, benzylpenicillin, vancomycin, doxycycline, amoxicillin-clavulanic acid and with decreased susceptibility to erythromycin and azithromycin [15].
Methodology of this study followed the standard Agar Diffusion Test (ADT). ADT is used for evaluating the antibacterial properties of root canal irrigants and intracanal medicaments. ADT is based on placing specimens on agar plates which are seeded with microorganisms. After incubation, the evaluation of degree of antibacterial activity around the specimens is taken by the gauge of inhibition zone [16]. ADT done in this study is more consistent with other studies, for testing the ability of antimicrobial action [17]. The results of this invitro study demonstrated that all the five irrigating solutions have shown zones of inhibition and there was statistically significant difference between five irrigating solutions in terms of zones of inhibition.
In the present study, larger zone of inhibition was seen with 30mcg augmentin in comparison with 5mcg sparfloxacin, 2% CHX, 3% NaOCl and 5% NaOCl. Augmentin is the most commonly prescribed antibiotic during endodontic treatment. Amoxicillin combined with clavulanic acid has high antibacterial activity against E. faecalis [13]. In a study conducted by Scukaite et al., predominant endodontic pathogens isolated from teeth with symptomatic apical periodontitis were highly sensitive to amoxicillin and amoxicillin is an antibiotic of choice during treatment of endodontic infections when conventional endodontic treatment is insufficient [18]. Salian Shailaja et al., reported that Co-amoxiclav combined with Citric acid and polysorbate-80 have shown better antibacterial activity than 2% CHX [19].
In the present study, 5mcg Sparfloxacin had shown better antimicrobial efficacy than 2% CHX, 3% NaOCl and 5% NaOCl. Many studies reported that Sparfloxacin is superior when compared to ciprofloxacin, ofloxacin, cefazolin, doxycycline against gram-positive bacteria [5]. In a study conducted by V. Parthasarathy et al., sustained release system of sparfloxacin (Sparfloxacin Chip) was used for the treatment of chronic generalized periodontitis which resulted in the complete eradication of the pathogenic bacteria from the periodontal pockets [20].
2% CHX has wide range of activity against both gram-positive and gram-negative bacteria. It adsorbs into dental tissue and mucous membrane, resulting in gradual release at therapeutic levels [21]. In a study conducted by Gomes et al., CHX tested in concentrations 0.2%, 1%, and 2% was as effective as 5.25% NaOCl in killing E.faecalis [22].
NaOCl is the most widely used irrigating solution. Siqueria et al., reported that NaOCl showed superior antibacterial effect against E.faecalis when compared with 0.9% NaCl solution. E.faecalis is resistant to NaOCl at lower concentrations whereas higher concentrations of NaOCl are undesirable as it is an irritant to periapical tissues. Antimicrobial activity of NaOCl was related to its concentration i.e., lower concentrations took more time to inhibit bacterial growth when compared with higher concentrations [23].
Till-date, local antibiotics were used as temporary canal dressings for root canal disinfection; they didn’t become a part of root canal disinfection and microbial eradication. There are various reasons for the failure of antibiotics to be used as root canal disinfectants. As antibiotics tested are bacteriostatic, which could just prevent the growth of microorganisms rather than killing them and finally giving the host defense to deal with the microbial infection, whereas in necrotic root canals, as there is lack of circulation there is no host defense. Finally the outcome of local administration of antibiotics in root canals may be only temporary. Higher concentrations of locally used antibiotics have a bactericidal effect, most effective when there is active growth phase of microbial cells, whereas in necrotic root canals with limited nutrients it may not be applicable. In Endodontics, there is limited information available about the effectiveness of intracanal antibiotics in infection control. Results of the present study may not be directly extrapolated to clinical situation. In the present study, there are several limitations which cannot be directly extrapolated to clinical conditions, which include penetration of antibiotics into dentinal tubules, selectivity for host cells, toxicity and the allergic potential of the experimental antibiotics was not taken into consideration [24]. Microbial inhibition potential of augmentin and sparfloxacin observed in this study opens perspectives for their use as intracanal irrigating solutions. However, preclinical, clinical trials and further research are required to evaluate biocompatibility and safety before Augmentin and Sparfloxacin can conclusively be recommended as root canal irrigating solutions.
Conclusion
This was a preliminary study of antimicrobial efficacy of two experimental antibiotics as irrigants against E.faecalis. Within the limitations of this study, 5mcg Sparfloxacin and 30mcg Augmentin have shown effective antimicrobial efficacy against E.faecalis. 2% CHX, 3% NaOCl and 5% NaOCl had an observable effectiveness against E.faecalis but showed comparatively less antimicrobial efficacy than 5mcg Sparfloxacin and 30mcg Augmentin.