Low Level Laser Therapy in the Treatment of Intra-Osseous Defect- A Case Report
Smiti Bhardwaj1, Joann Pauline George2, Divakaran Remigus3, Divya Khanna4
1 Research Associate, Department of Implantology, Maulana Azad Institute of Dental Sciences, New Delhi, India.
2 Professor, Department of Periodontology, Krishnadevaraya College of Dental Sciences & Hospital, Bangalore, Karnataka, India.
3 Post Graduate Student, Department of Periodontology, Krishnadevaraya College of Dental Sciences & Hospital, Bangalore, Karnataka, India.
4 Post Graduate Student, Department of Periodontology, Krishnadevaraya College of Dental Sciences & Hospital, Bangalore, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Joann Pauline George, Professor, Department of Periodontology, Krishnadevaraya College of Dental Sciences & Hospital, Hunsamaranhalli, New Airport Road, Bangalore, Karnataka-562157, India.
E-mail: drjoannpaulinegeorge@gmail.com
Low level laser has been documented in literature to promote wound healing by reducing postoperative inflammation, oedema and reduces pain. This case report demonstrates the safe and positive outcome of LLLT in conjunction with demineralised bone matrix of bovine origin in the surgical treatment of a periodontal infrabony defect. After surgical defect debridement, low level semiconductor diode laser (GaAlAr) was delivered for 5 minutes to the inner margins of flap in contact mode and the defect was irradiated with LLLT in a non-contact mode for 10 minutes. Demineralised bone matrix graft was used as a bone grafting material. Repeat irradiation was done for the next 5 days on the outer buccal and lingual flap surfaces. A CAL gain of 4 mm and 37% bone fill was noted radiographically at end of 12 months. LLLT can be used as an adjunct to periodontal regeneration.
Bone Regeneration, Bone Substitutes, Diode laser, Laser Therapy, Low- Level Laser
Case Report
A 45-year-old male patient reported to the Department of Periodontology, Krishnadevaraya College of Dental Sciences and Hospital, Bangalore for management of dull and nagging pain since 4 days in 45 and 46. Intraoral examination revealed generalized periodontal pockets (4-7 mm), Grade II furcation involvement in 46 and localized periodontal abscess in respect to 44 and 45. Radiographically, 44 and 45 demonstrated moderate angular bone loss with a deeper component distally. The case was diagnosed as moderate chronic periodontitis with chronic localized periodontal abscess at 44 and 45. A comprehensive treatment plan including non-surgical and presumptive surgical therapy including use of LLLT and bone graft was presented before the patient and signed informed consent was obtained.
On day 1, intra-sulcular emergency abscess drainage was carried out at 44 and 45. Non-surgical periodontal therapy consisting of full mouth scaling and root planing was completed within next three weeks, with follow-up up of three months. The clinical parameters were recorded [Table/Fig-1,2]. Observing optimal plaque control, good patient compliance and deep residual pockets, decision for surgical treatment was made and informed to the patient.
The clinical measurements at baseline, six months, and 12 months.
| Clinical Attachment Level | Gingival Recession | Probing Pocket Depth |
|---|
| DB | B | MB | DL | L | ML | DB | B | MB | DL | L | ML | DB | B | MB | DL | L | ML |
|---|
| Baseline | 10 | 2 | 3 | 8 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 2 | 3 | 8 | 2 | 2 |
| 6 Months | 7 | 2 | 1 | 7 | 2 | 1 | 3 | 2 | 1 | 2 | 2 | 1 | 4 | 2 | 2 | 3 | 2 | 2 |
| 12 Months | 6 | 2 | 2 | 4 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 4 | 2 | 2 | 2 | 2 | 2 |
| Diff B/wBaseline and12 months | 4 | - | 1 | 4 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 6 | - | 1 | 6 | - | - |
Measured in mm
The radiological measurements at baseline, six months and 12 months.
| BASELINE | 6 MONTHS | 12 MONTHS |
|---|
| DEFECT ANGLE (DEGREE°) | 68° | 66° | 32° |
| CEJ – Base of defect | 6.7 | 6.0 | 4.2 |
| Linear Bone Growth | — | 0.7mm | 2.5mm |
| % Bone fill | — | 10% | 37% |
Measured in mm
Using # 12 stainless steel surgical blade, initial crevicular incisions were placed and the buccal and lingual mucoperiosteal flaps were carefully reflected using #20 periosteal elevator. A two wall osseous defect (lingual and distal) coronally and three-wall defect apically was observed. The defect in between 44 and 45 was debrided with a combined use of area specific curettes and roots were carefully planed using ultrasonic power driven instruments. At the end of instrumentation, 24% ethylene-diamine-tetracetic acid (EDTA) was applied on the instrumented root surface for three minutes [1]. The defect area was carefully rinsed with saline and meticulously isolated from blood and saliva in order to prevent contamination.
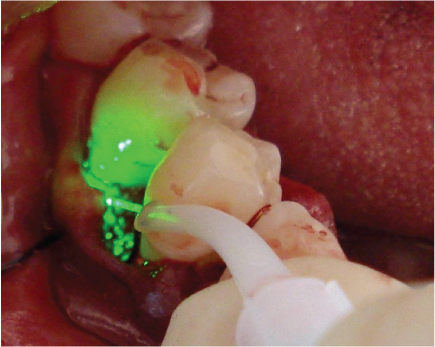
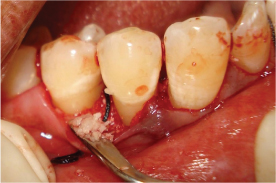
After optimal and careful surgical defect and root debridement, semiconductor diode laser (GaAlAr) with wavelength 810 nm, output power 100 mW at 4J/cm2 was delivered for 5 minutes, in continuous wave contact mode to the inner margins of flap at an angle of 45 degrees [Table/Fig-3] [2]. Later, the defect was irradiated with LLLT in continuous non-contact mode for 10 minutes [3]. To ensure uniform exposure of the whole defect region, the probe Was positioned in contact mode with the flaps from the margins to the centre of wound in circular motion. Pre-suturing was performed and a demineralised bone matrix (DBM) osteoinductive xenogenic bone graft material was used to sequentially fill the defect site [Table/Fig-4]. Vertical mattress sutures were placed.
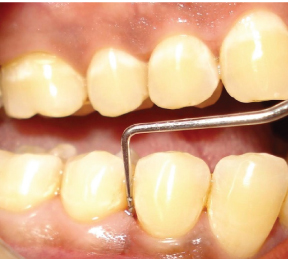
Probing depth at distobuccal site of mandibular right first premolar – baseline.
Low level laser therapy application.
The patient was advised to take Ibuprofen 400 mg for pain relief thrice daily till the time pain was experienced. The patient was recalled for next five days consecutively and LLLT was performed for 5 min from outer surfaces of buccal and lingual flaps. A protocol for the control of bacterial contamination consisting of Doxycycline (200 mg loading dose and 100 mg OD for 1 week) and 0.12% chlorhexidine mouth rinsing three times daily was prescribed. Patient was instructed to avoid brushing, flossing and chewing in the treated area for a period of two weeks after which, he was asked to resume his oral hygiene procedures. The patient was placed on a three month recall and was followed up to 12 months. Clinical attachment level (CAL) measurements were made using cemento-enamel junction as reference point.
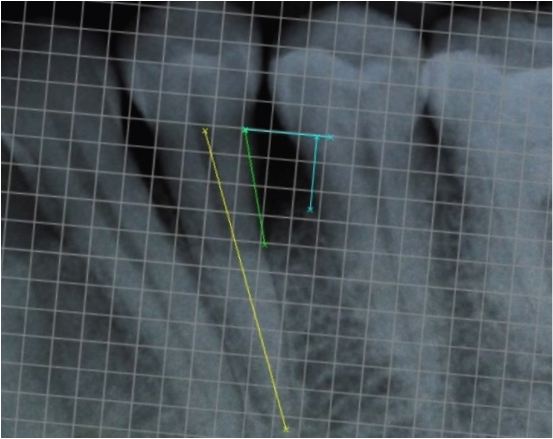
Patient reported no pain and swelling at the surgical site, two days after the surgery. Healing was uneventful. Patient discontinued the use of analgesics after the second day; however, the antibiotic was continued as prescribed. The six months and 12 months follow up showed improvements [Table/Fig-2,5]. The oral hygiene maintenance of patient was adequate with full-mouth plaque of 0.98. The presurgical and postsurgical radiographs were analysed using digital image analysis software, with a mm grid scale and the following measurements were made - CEJ to base of the bone defect, CEJ to the alveolar crest, CEJ to the root apex [Table/Fig-6,7]. The most coronal portion where the periodontal ligament maintained a uniform width was considered as the base of the defect and the crossing of the silhouette of the alveolar bone with the root was considered as the alveolar crest. Linear bone growth (LBG) was calculated as CEJ to base of the bone defect at baseline- CEJ to base of the bone defect at 12 months. Percentage bone fill was calculated by dividing LBG by the distance of CEJ to the base of the defect.
Demineralised bovine graft placement.
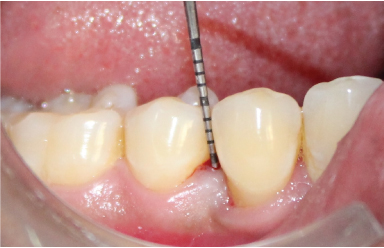
Probing depth at 12 months.
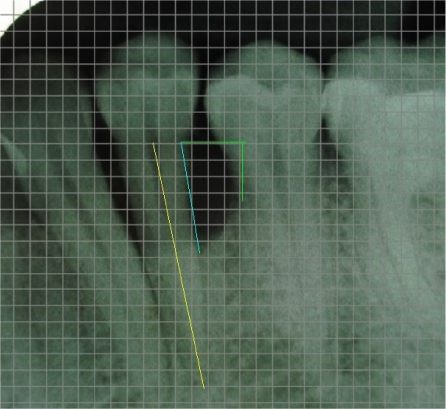
Radiographically, a Linear Bone Growth (LBG) of 2.5mm and 37% bone fill was observed at the infrabony defect distally [Table/Fig-8] at the 12 months follow up. The radiographic defect angle of intrabony defects was measured using the three anatomical landmarks, the cemento-enamel junction, the alveolar crest and the base of the bone defect [Table/Fig-6]. The angle of the defect decreased from 68° at baseline to 32°, by the end of 12 months [Table/Fig-6,7].
Discussion
LLLT promotes wound healing and reduces pain after gingivectomy, non-surgical and surgical periodontal treatment [4,5]. LLL can enhance bone regeneration by enhancing proliferation and maturation of human osteoblasts [6,7]. There are however no reports on the use of LLLT in conjunction with bovine derived bone grafts for periodontal regeneration. The present case report thus discusses for the first time the use of LLLT in conjunction with bovine derived demineralised bone matrix.
This is the first case report that reports the use of LLLT with DBM in the treatment of intrabony defects. In the current case report, there was an uneventful healing with LLLT and DBM (osteoinductive, bone graft material) in the treatment of periodontal intrabony defect. A CAL gain of 4 mm, linear bone gain of 2.5mm, bone fill of 37 % and defect angle reduction from 68o to 32o noted at 12 months follow up shows a positive outcome of the treatment. There are no studies with LLLT and DBM to compare the outcome but in a similar study a statistically significant improvement in CAL gain was observed when guided tissue regeneration was performed with equine bone + membrane + LLLT [8] and also with LLLT + bioactive glass [9] in periodontal regeneration.
There was satisfactory soft tissue healing at the site of the surgery, with minimal oedema and pain at day 2. The results are similar to that noted by Ozcelik [10] when EMD + LLLT was used in the treatment of infrabony defects. He noted reduced oedema, less recession and less pain. In the present case similarly minimal recession and less pain was noted. Reduced pain has been attributed to selective inhibition of nociceptive signals arising from peripheral nerves and reduced inflammation [5].
LBG of 2.5mm and 37% bone fill demonstrates a positive outcome of the treatment approach. A combined effect of the osteoinductive graft material, LLLT and surgical access flap was observed clinically and radiographically. Osseograft™ is an osteoinductive, xenogenic, demineralised bone matrix (DBM), and derived from bovine bone. It is composed of Type I collagen contrived in particulate form with particulate size of 250 μm. Once the DBM is placed in the osseous defect, a sequential differentiation of mesenchymal-type cell occurs promoting osteoblastic proliferation and differentiation. LLLT induces faster cell division, proliferation rate, migration of fibroblast and rapid matrix production [11]. LLLT increases local blood flow, enhancing the supply of circulating cells, nutrition, oxygen and inorganic salts to the bone defect [12].
LLLT may improve the regenerative effects by stimulating osteoblastic cell differentiation from progenitor stem cells [12]. Application of mineral bone graft together with laser irradiation is associated with higher defect healing than using bone graft only [3]. Laser irradiation significantly increased the number and the total area of bone nodules in a dose-dependent manner while osteoblastic differentiation [7]. An increase in bone micro hardness has been reported in LLLT group over control groups [6]. LLLT at 4 J/cm2 is effective in bone formation, especially in fourth week [3]. In the present case report, semiconductor diode laser (GaAlAr) with wavelength 810 nm at 4J/ cm2 was used toIrradiate the defect and positive result was encountered. Complete bone regeneration with laser application at 830 nm as compared with 660 nm has been demonstrated [3].
Repeated laser applications are necessary to achieve a positive effect on the proliferation of fibroblasts and hence, in the present case, LLLT was repeated for five consecutive days after the surgical procedure. This accounted for a total of 75 minutes of LLLT tissue exposure [3].
Conclusion
The combined approach of LLLT and demineralized bone matrix of bovine origin showed a postitive outcome with CAL gain, reduction in Periodontal Probing Depth (PPD), minimal recession clinically and Linear bone gain and bone fill radiographically. This combination approach is safe and can be used for periodontal regeneration. LLLT can be used as a safe and effective adjunctive therapy with conventional surgical therapies aiming at accelerated healing along with periodontal regeneration. However, randomized clinical trials and histological studies are required in the future to further confirm the outcomes reported in this paper.
Measured in mm
Measured in mm
[1]. Gamal AY, Enhanced beta-tricalcium phosphate blended clot adhesion to EDTA biomodulated periodontally affected root surfaces: in vivo scanning electron microscopy evaluationJ Periodontol 2011 82(11):1587-95. [Google Scholar]
[2]. Sanz-Moliner JD, Nart J, Cohen RE, Ciancio SG, The effect of an 810-nm diode laser on postoperative pain and tissue response after modified Widman flap surgery: a pilot study in humansJ Periodontol 2013 84(2):152-58. [Google Scholar]
[3]. Rasouli Ghahroudi AA, Rokn AR, Kalhori KA, Effect of low-level laser therapy irradiation and bio-oss graft material on the osteogenesis process in rabbit calvarium defects: a double blind experimental studyLasers Med Sci 2014 29(3):925-32. [Google Scholar]
[4]. Amorim JC, de Sousa GR, de Barros Silveira L, Clinical study of the gingiva healing after gingivectomy and low-level laser therapyPhotomed Laser Surg 2006 24(5):588-94. [Google Scholar]
[5]. Qadri T, Miranda L, Tuner J, Gustafsson A, The short-term effects of low-level lasers as adjunct therapy in the treatment of periodontal inflammationJ Clin Periodontol 2005 32(7):714-19. [Google Scholar]
[6]. Da Silva AP, Petri AD, Crippa GE, Effect of low-level laser therapy after rapid maxillary expansion on proliferation and differentiation of osteoblastic cellsLasers Med Sci 2012 27(4):777-83. [Google Scholar]
[7]. Stein A, Benayahu D, Maltz L, Oron U, Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in vitroPhotomed Laser Surg 2005 23(2):161-66. [Google Scholar]
[8]. Dogan GE, Demir T, Orbak R, Effect of low-level laser on guided tissue regeneration performed with equine bone and membrane in the treatment of intrabony defects: a clinical studyPhotomed Laser Surg 2014 32(4):226-31. [Google Scholar]
[9]. AboElsaad NS, Soory M, Gadalla LM, Effect of soft laser and bioactive glass on bone regeneration in the treatment of infra-bony defects (a clinical study)Lasers Med Sci 2009 24(3):387-95. [Google Scholar]
[10]. Ozcelik O, Cenk Haytac M, Seydaoglu G, Enamel matrix derivative and low-level laser therapy in the treatment of intra-bony defects: a randomized placebo-controlled clinical trialJ Clin Periodontol 2008 35(2):147-56. [Google Scholar]
[11]. Kreisler M, Christoffers AB, Al-Haj H, Willershausen B, d’Hoedt B, Low level 809-nm diode laser-induced in vitro stimulation of the proliferation of human gingival fibroblastsLasers Surg Med 2002 30(5):365-69. [Google Scholar]
[12]. Wu JY, Chen CH, Yeh LY, Low-power laser irradiation promotes the proliferation and osteogenic differentiation of human periodontal ligament cells via cyclic adenosine monophosphateInt J Oral Sci 2013 5(2):85-91. [Google Scholar]