T2DM is an independent risk factor for cardiovascular disease and is associated with increased susceptibility to cardiovascular complications. Cardiovascular disease is the predominant cause of death in diabetic patients. It has been hypothesized that control of plasma glucose levels would reduce cardiovascular disease in T2DM thus lowering parameters such as mortality rate, myocardial infarction or stroke.
Recently, dipeptidyl peptidase-IV (DPP-IV) inhibitor, the new class of anti-diabetic drug which having a unique mechanism of action has been introduced in the market. DPP-IV inhibitors act via Glucagon-like peptide 1 (GLP-1), resulting in lowering of blood glucose levels. GLP-1 is an incretin hormone that facilitates glucose-induced insulin release. In isolated cardiac myocytes, GLP-1 has demonstrated chronotropic effects [1]. Studies using GLP-1 receptor knockout mice have also suggested a role for the GLP-1 receptor in the control of cardiac structure and function [2]. Administration of GLP-1 improved left ventricular function in patients with acute myocardial infarction and left ventricular dysfunction [3]. Native GLP-1 has a short half-life of minutes, being rapidly degraded by DPP-IV. DPP-IV inhibitors prolong the circulating half-life of GLP-1, thus making DPP-IV inhibitors a promising target for the treatment of type 2 diabetes with co-existing ischemic heart disease. The marketed synthetic DPP-IV Inhibitors are relatively expensive compared to standard drugs like Metformin. Moreover, marketed synthetic DPP-IV Inhibitors has been reported to cause unacceptable adverse effects like acute pancreatitis, pancreatic cancer, thyroid cancer as well as reduce immune function [4–6].
In the current scenario, if we could have DPP-IV Inhibitors, from the natural sources that share the beneficial effects of DPP-IV Inhibitors but are devoid of the unacceptable adverse effects and at the same time are less expensive as well as work in concert with the body’s defense mechanism. Berberine, an alkaloid isolated from the root of Berberis aristata has shown to display a wide array of pharmacological activities: antimicrobial, anti-diabetic, anti hyperlipidemic, anti-inflammatory, wound healing and hepatoprotective [7,8]. Al-masri et al., reported the DPP-IV Inhibitory activity of berberine [9]. In addition studies have shown that berberine is beneficial in combating cardiovascular disease [10]. Therefore, berberine may represent a novel therapeutic target and strategy for diabetes with co-existing cardiovascular complication.
With the point of view that it might be interesting and possibly fruitful to study the cardioprotective effects of berberine in the setting of diabetes mellitus challenged with Isoproterenol induced myocardial injury, the present study was designed. In addition, the underlying mechanism of their beneficial therapeutic effects has been evaluated.
Materials and Methods
Streptozotocin (STZ) and Isoproterenol (ISP) were procured from Sigma Chemicals St. Louis, USA. The test drug Berberine was also procured from Sigma Chemical, St. Louis, USA. All other chemicals and reagents were used of analytical grade.
Experimental Animals
The study was approved from the Institutional Animal Ethics Committee. This is experimental study conducted in Department of Pharmacology, MGM Medical College, Navi Mumbai between Feb 2015 to March 2015. Wistar male mature rats, weighing 150 to 200g, 10 to 12-week-old were used in the study. The animals were procured from the Bombay Veterinary College and were housed in polyacrylic cages. The animals were acclimatized for one week before the commencement of the experiment. The rats were maintained under standard laboratory conditions under natural light and dark cycles (approximately 14 h light/10 h dark) and an ambient temperature of 25±2o C. The animals were allowed free access to standard pellet diet and tap water ad libitum.
Experimental Procedure
All animals except healthy control group rats were allowed free access to normal rat diet and the rats were be injected intraperitoneally with streptozotocin (STZ) (45 mg/kg). Blood glucose, was estimated 7 days after the STZ injection. The rats with blood glucose of ≥200 mg/dl, was considered diabetic rats and selected for further pharmacological studies. These rats were randomly allocated to various experimental groups and fed test drug orally using a feeding cannula for next 30 days.
Treatment Protocol
The animals were randomly allocated into three main groups comprising of six animals each.
Group 1 — Healthy control group — Healthy Control.
Rats were administered Distilled water once daily for a month and then sacrificed.
Group 2 — Diabetic control group — Diabetic Control.
The animals were administered Streptozotocin (45mg/kg) on 1st day and in addition received ISP (85 mg/kg, sc) on 35th and 36th day at an interval of 24 h .
Group 3 — Berberine Treated Group — Berberine treated.
The Berberine (100 mg/kg) were fed orally to diabetic rat from 8th day to 37th day daily and in addition received ISP (85 mg/kg, sc) on 35th and 36th day at an interval of 24 hour.
On the 37st day the animals were sacrificed under ketamine anaesthesia for biochemical studies. The pancreas, hearts, liver and kidney were excised and immediately processed for histopathological studies.
Parameter Evaluated
Changes in Body Weight
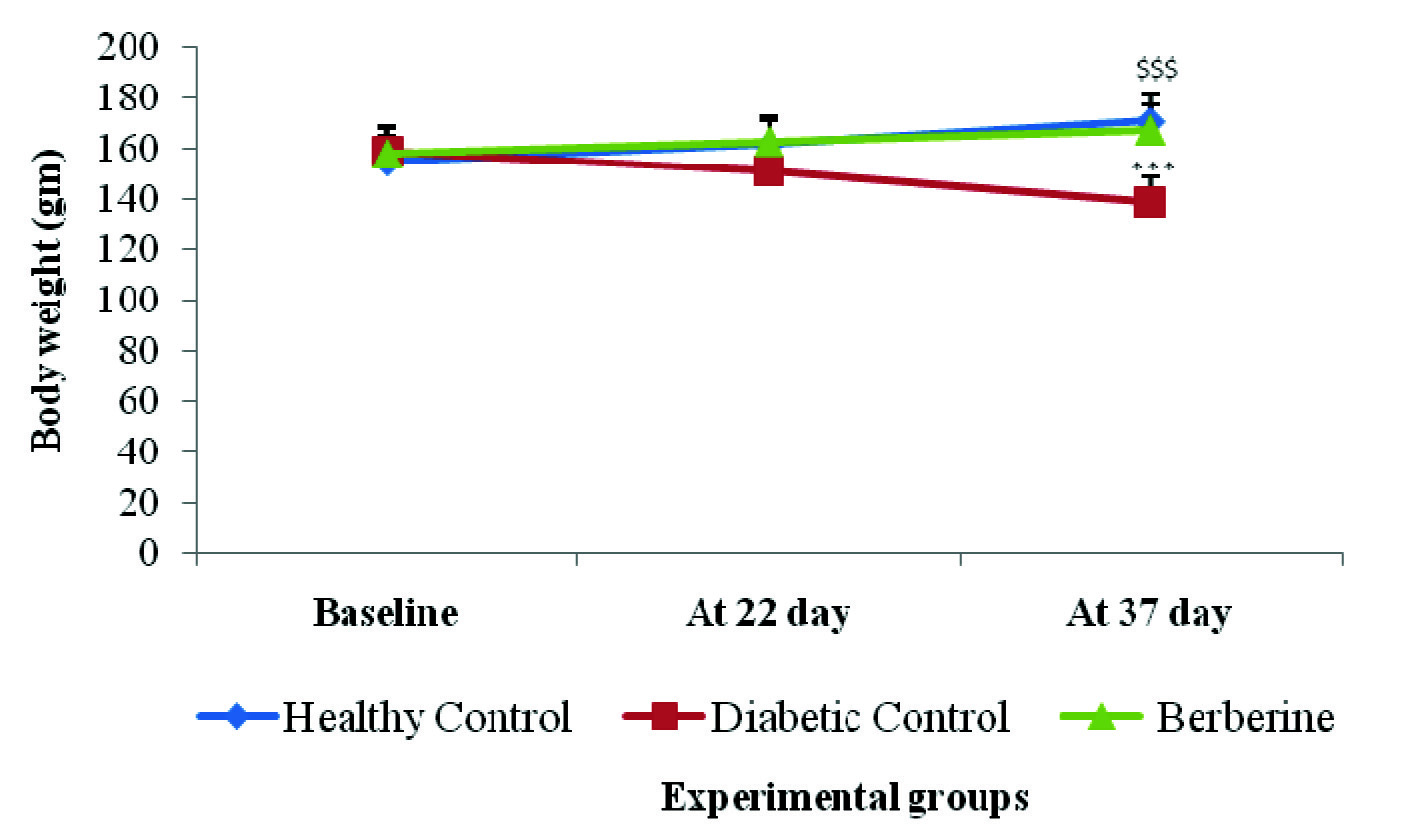
The body weight (gm) of all group rats was recorded every 15 days and the change in body weight was calculated. At the same time mortality if any during the experimental duration with the respective drugs was also be monitored.
Estimation of Biochemical Parameters
The rat blood sample of all experimental group was collected from the retro-orbital plexsus under light anaesthesia at 0, 7, 22 and 37 days for estimation of blood glucose. In addition, after the completion of the experimental duration (37 days), serum was used for the determination of the anti-diabetic (Glucose, HbA1c), cardioprotective (CPK-MB), metabolic (lipid profile), safety {liver function (SGPT, kidney function (Creatinine)} parameters by Auto-analyser in the Pathology (NABL accredited) and Pharmacology laboratory.
Histopathological studies
At the end of the experiment (37 days), the animals were sacrificed. The heart, liver, kidney and pancreas was immediately be fixed in 10% buffered neutral formalin solution. The tissues were carefully embedded in molten paraffin with the help of metallic blocks, covered with flexible plastic moulds and kept under freezing plates to allow the paraffin to solidify. Cross sections (5 μm thick) of the fixed tissues were cut. These sections were stained with haematoxylin and eosin and visualized under microscope to study the microscopic architecture of the tissues. The investigators performing the histological evaluation was blind to biochemical results and to treatment allocation.
Statistical Analysis
Descriptive statistics such as mean and standard deviation were calculated for all variables for each group. Student ‘t’ test was applied for statistical analysis of the biochemical parameters. The p-value <0.05 was considered as statistical significance level.
Results
Confirmation of Type 2 Diabetes and myocardial necrosis in rats.
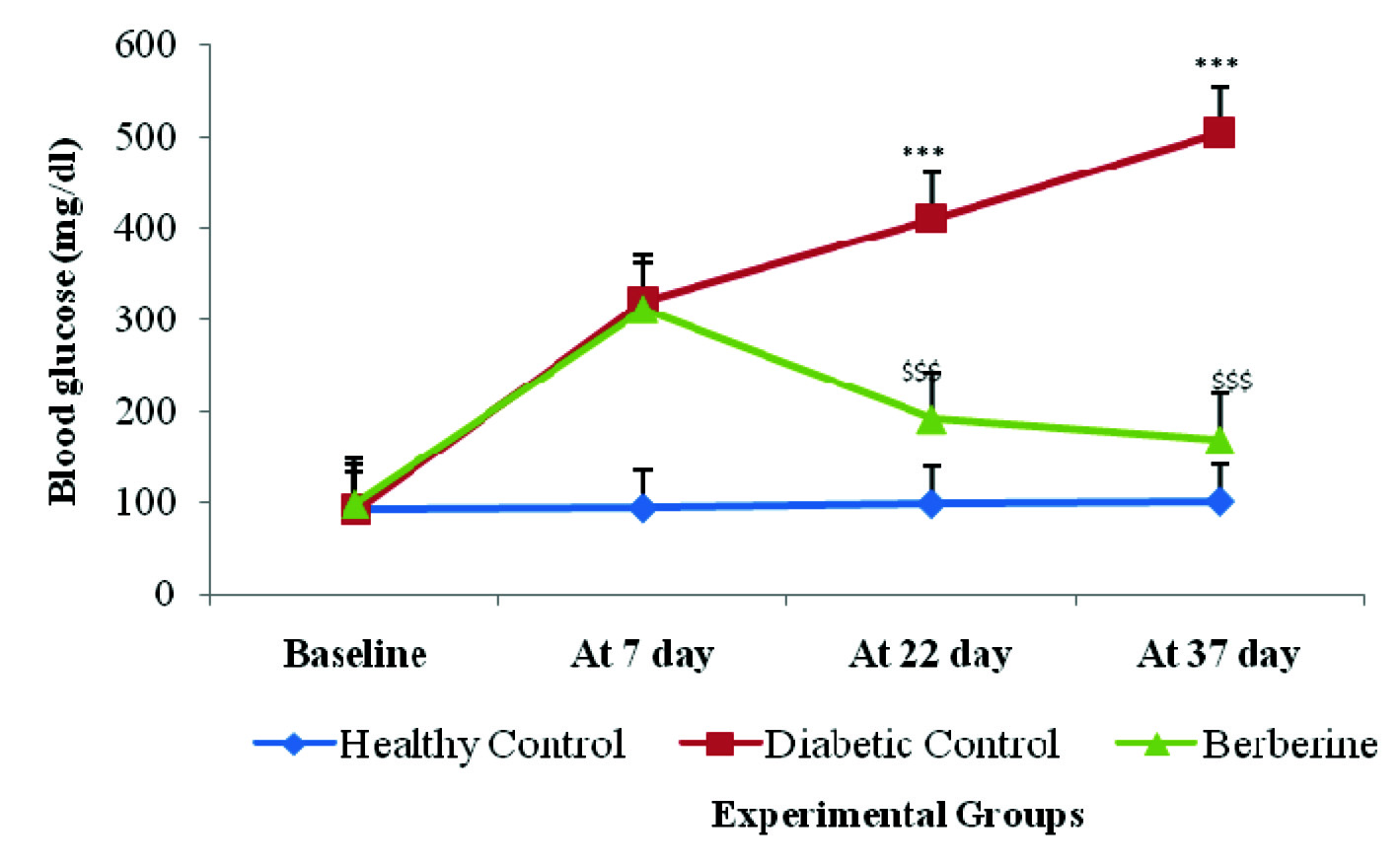
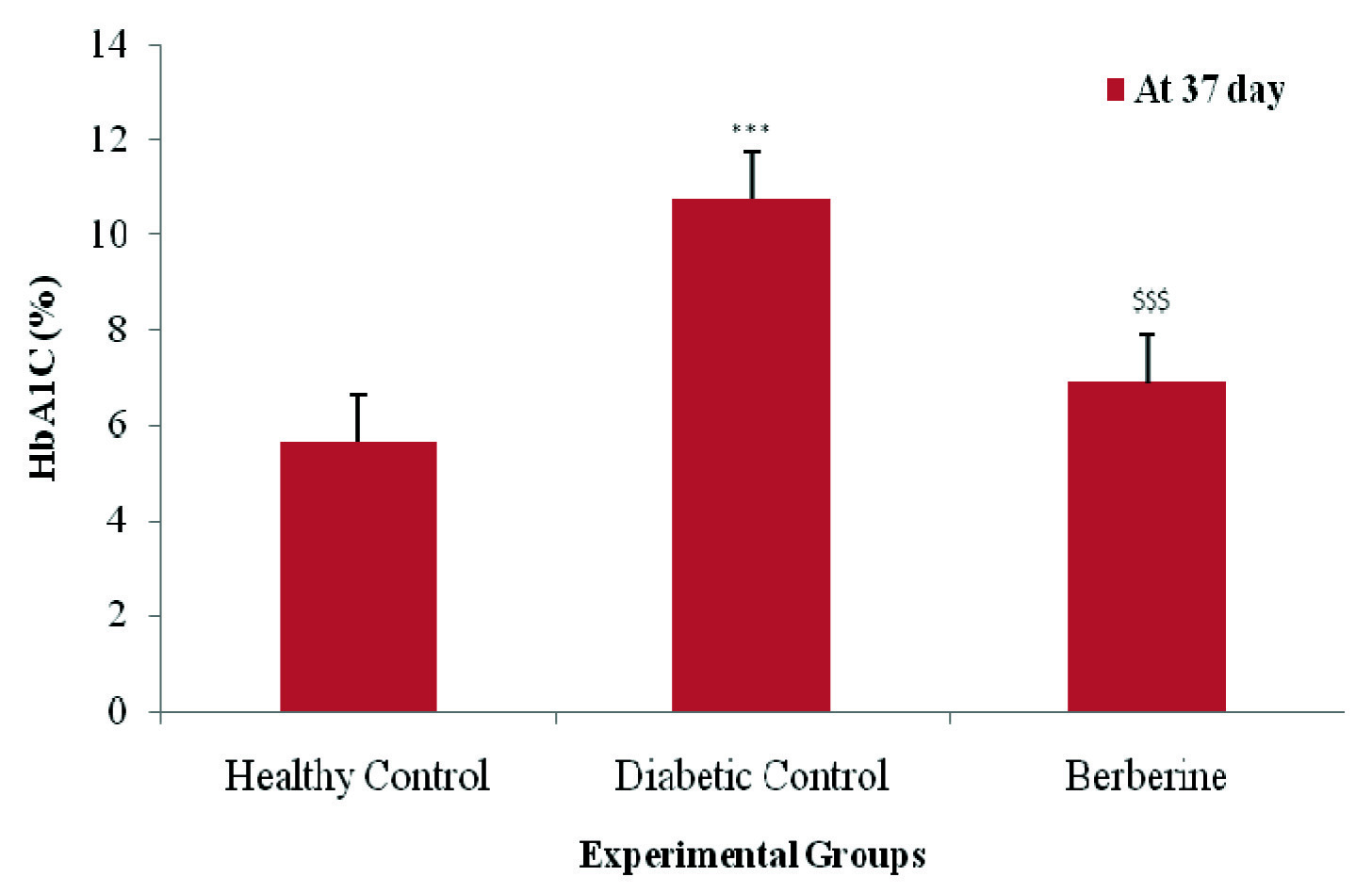
Single intraperitoneal (i.p) injection of Streptozotocin (45mg/kg) to rats produced severe hyperglycemia (320.5±7.78) and increased HbA1c level (10.75±0.21) in the animals. In addition, increased cardiac marker CPK-MB in the diabetic control group, produced by injecting (S.C.) Isoproterenol 85mg/kg, confirmed myocardial infarction [11].
Effect of Berberine on Body weight (gm)
In comparison to healthy control group, in diabetic control groups rats a decrease in weight (p<0.001) at 22, 37 day was observed. Berberine treated group restored (p<0.001) body weight at 22, 37 days respectively as compared to diabetic control group rats [Table/Fig-1].
Effect of Berberine on Blood glucose (mg/dl) and HbA1c (%)
Blood glucose was measured with glucometer. There was a significant (p<0.001) increase in blood glucose and HbA1c levels in diabetic control group rats as compared to healthy control group rats. Oral feeding of berberine treatment significantly restored (p< 0.001) the elevated blood glucose and HbA1c levels as compared to the diabetic control group rats at 37 day [Table/Fig-2,3].
Effect of Berberine on Cardiac marker CPK-MB (U/L)
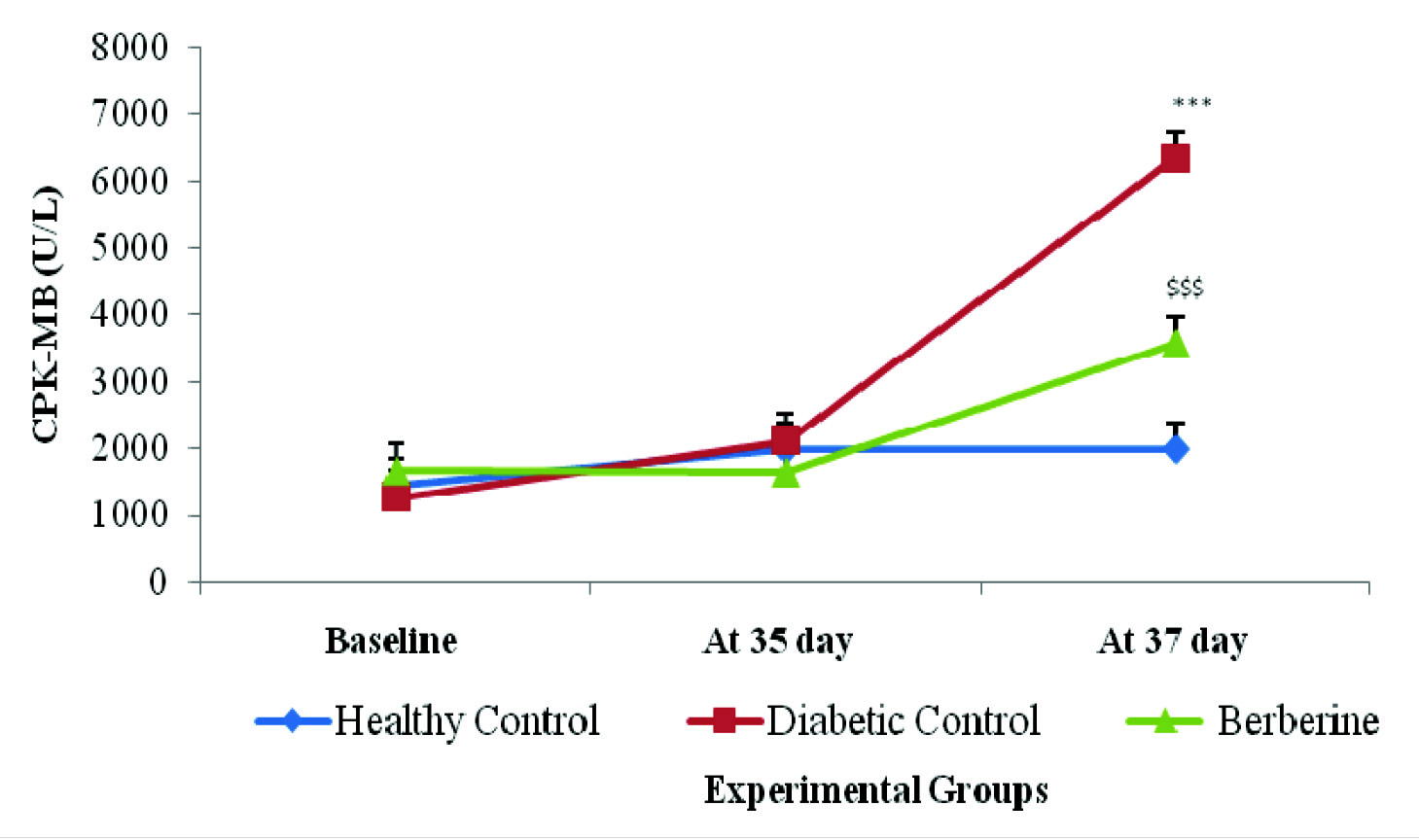
There was a significant (p<0.001) increase in serum CPK-MB level in diabetic control group rats as compared to healthy control group. Treatment with Berberine significantly (p<0.001) reduced elevated serum CPK-MB levels in berberine treated group as compared to diabetic control group respectively. In the healthy control group, at 37th day, CPK-MB was found to be 1985.4±1145.6 U/L which increased to 6351±779.23U/L in the diabetic control group. Treatment with berberine restored this significant rise in CPK-MB values as compared to diabetic control (3567.5±1177.06 U/L) [Table/Fig-4].
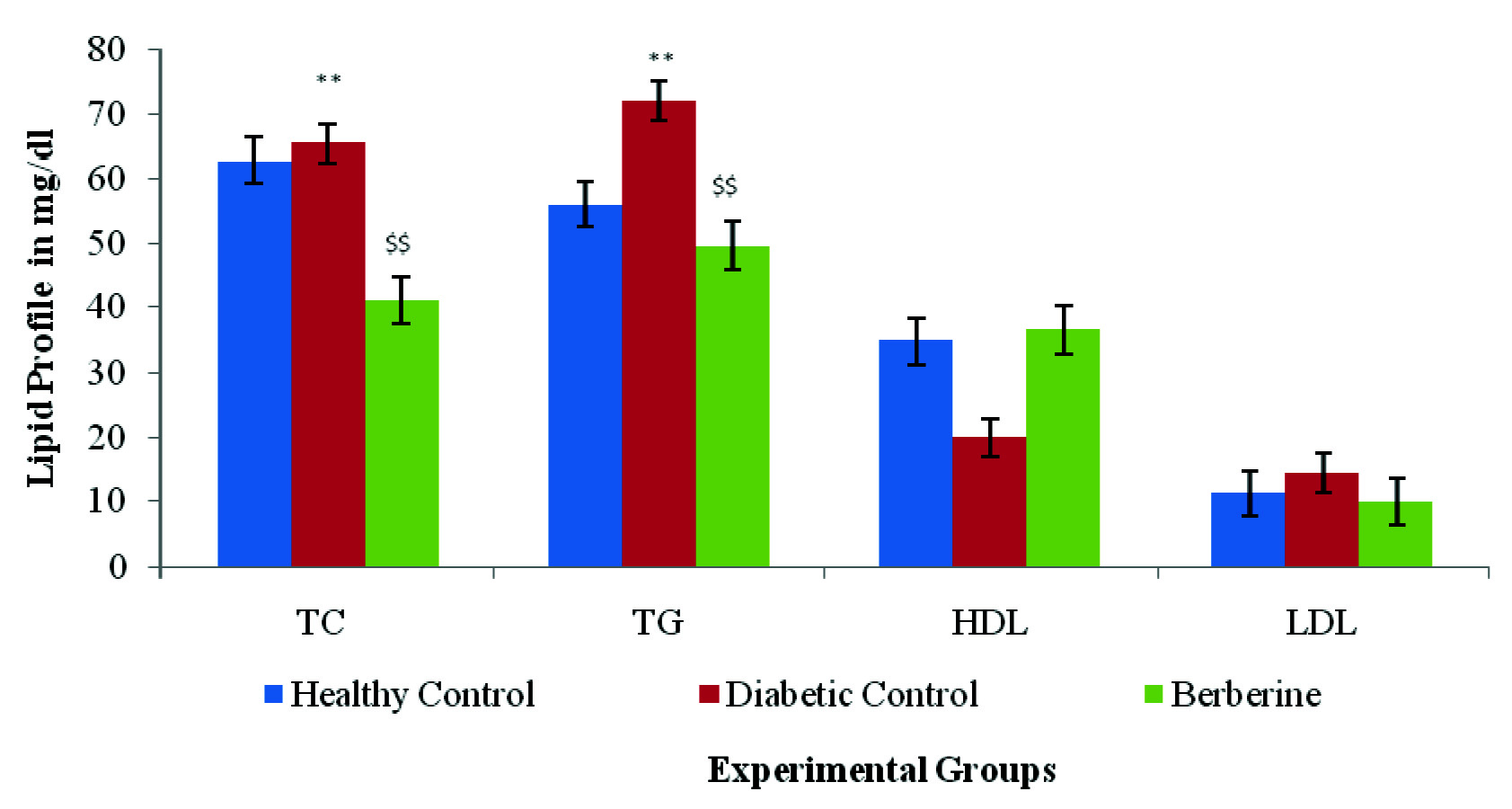
Effect of Berberine on Lipid Parameters (mg/dl)
[Table/Fig-5] depicts the serum levels of TC, TG, LDL-C and HDL-C in various experimental group. The diabetic control group rats showed significant (p<0.01) increase in serum TC, TG, LDL-C except HDL-C when compared with healthy control group of rats. Berberine treatment significant (p<0.01) restored the content of lipid markers (TC,TG, LDL, HDL) as compared with diabetic control group rats on 37th day [Table/Fig-5].
Effect of Berberine on safety parameters {Liver Function (SGPT), Kidney Function (Creatinine)}
The liver and kidney marker enzymes were analysed in the different experimental group. The diabetic control group rats showed a significant (p<0.001) increase in the level of SGPT (U/L) and creatinine (mg/dl) when compared to healthy control group rats on 37th day. The Berberine treated group showed (p<0.001) significant decrease in the level of liver and kidney marker enzymes when compared with diabetic control group on 37th day [Table/Fig-6].
Effect of berberine on body weight (gm) of healthy control, diabetic control, and berberine treated group. Values are expressed as mean±SD. (n = 6) ***p<0.001 Healthy control Vs diabetic control, $$$p<0.001 Diabetic control Vs Berberine.
Effect of berberine on Blood glucose (mg/dl) of Healthy Control, Diabetic Control, and Berberine treated group. Values are expressed as mean±SD. (n = 6) ***p<0.001 Healthy Control Vs Diabetic Control, $$$p<0.001 Diabetic Control Vs Berberine.
Effect of berberine on HbA1c value (%) of Healthy Control, Diabetic Control, and Berberine treated group. Values are expressed as mean±SD. (n = 6) ***p<0.001 Healthy Control Vs Diabetic Control, $$$p<0.001 Diabetic Control Vs Berberine.
Effect of berberine on CPK-MB value (U/L) of Healthy Control, Diabetic Control, and Berberine treated group. Values are expressed as mean±SD. (n = 6) ***p<0.001 Healthy Control Vs Diabetic Control, $$$p<0.001 Diabetic Control Vs Berberine.
Effect of berberine on Lipid profile (mg/dl) of Healthy Control, Diabetic Control, and Berberine treated group. Values are expressed as mean±SD. (n = 6) **p<0.01 Healthy Control Vs Diabetic Control, $$p<0.01 Diabetic Control Vs Berberine.
SGPT (U/L) {Serum glutamate Pyruvic transminase} and Creatinine (mg/dl): Values are expressed as mean±SD. (n = 6) ***p<0.001 Healthy Control Vs Diabetic Control, $$$p<0.001 Diabetic Control Vs Berberine.
| SN | Experimental groups | Liver Marker | Kidney Marker |
|---|
| SGPT (U/L) | Creatinine (mg/dl) |
|---|
| 1 | Healthy Control Group (n = 6) | 60.93±10.56 | 0.33±0.06 |
| 2 | Diabetic Control Group (n = 6) | 100.7±3.11(***) | 0.42±0.01(***) |
| 3 | Berberine treated Group (n = 6) | 61.21±7.59 ($$$) | 0.28±0.03 ($$$) |
Histopathological studies
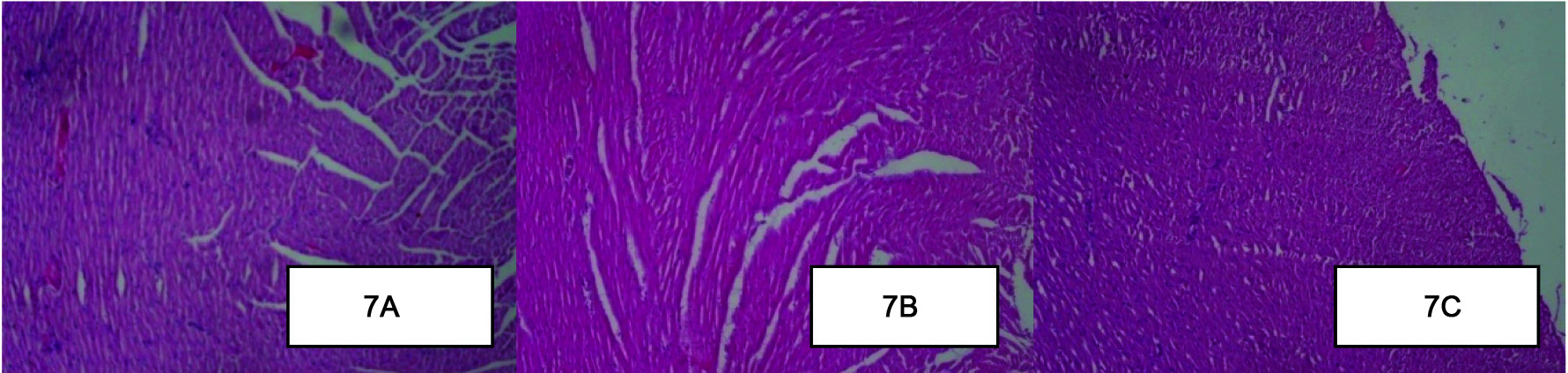
Effect of Berberine on Histopathology of the Myocardium
Histopathological assessment of healthy control group rat heart revealed the non-infarcted architecture of the myocardium [Table/Fig-7a]. In contrast, the diabetic control group rats subjected to STZ-ISP injury [Table/Fig-7b], demonstrated marked oedema, confluent areas of myonecrosis separation of myofibers, congested blood vessels and mild inflammation as compared to the healthy control group. In the Berberine treatment group rats [Table/Fig-7c], occasional focal myofiber loss, necrosis oedema and inflammation were observed. However, the degree of oedema, inflammation and necrosis was less as compared to the diabetic control group [Table/Fig-7a-c].
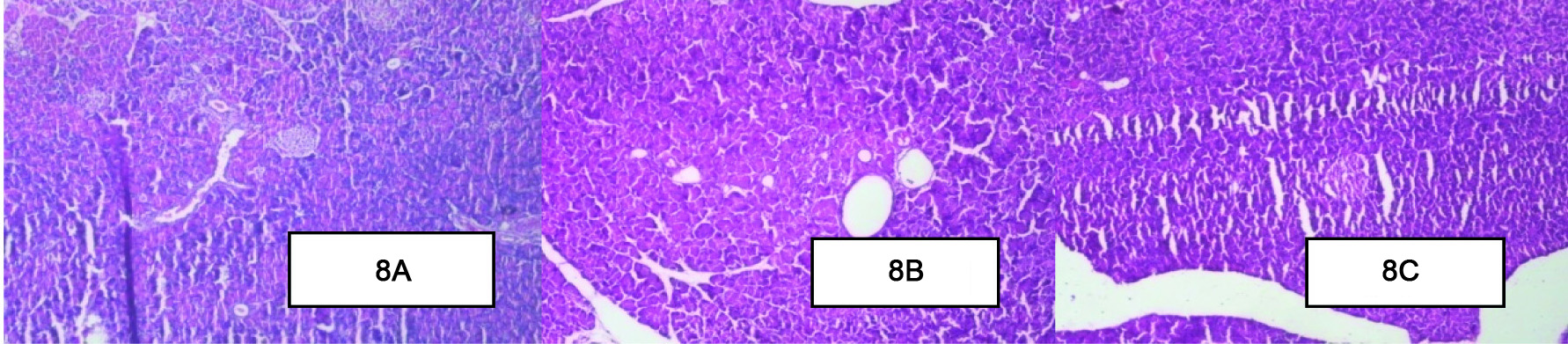
Effect of Berberine on Histopathology of the Pancreas
The pancreas of the healthy control group [Table/Fig-8a] of rats were characterized by an organized pattern and showed normal architecture of islets of langerhans and the beta cells. In contrast, the diabetic control group [Table/Fig-8b] of rats damaged islets of langerhans and the atrophy of beta cells with the loss of few nuclei and cytoplasm was observed. In berberine treated group [Table/Fig-8c] few nuclear changes were observed through less compare to diabetic control group [Table/Fig-8a-c].
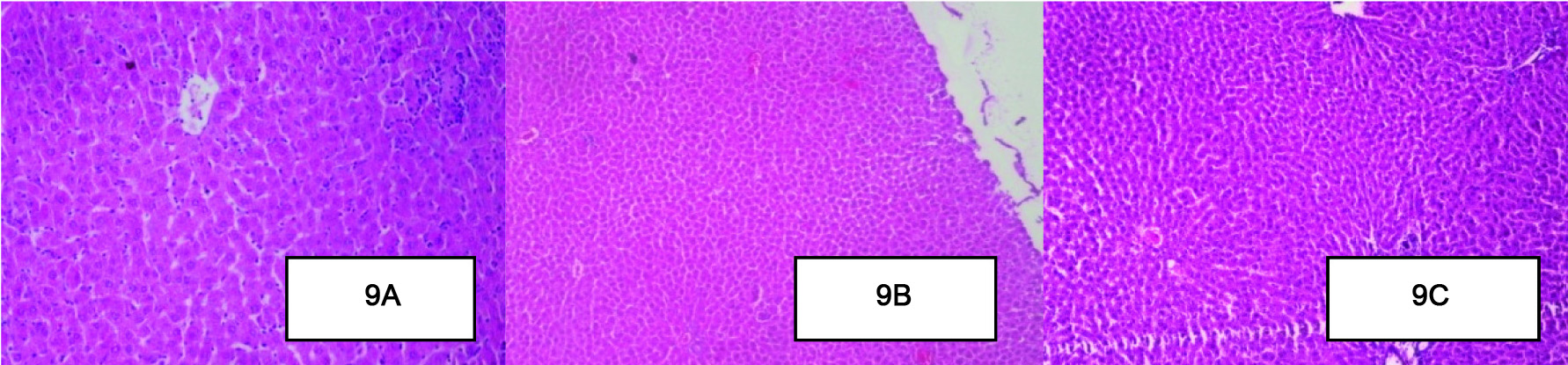
Effect of Berberine on Histopathology of the Liver
Histological assessment of the liver of the healthy control group [Table/Fig-9a] rats, shows normal architecture of central vein, peripheral vein and hepatocytes. In contrast, the liver of the diabetic control group [Table/Fig-9b] showed degeneration and scattered necrotic cells. However, in the berberine treated group [Table/Fig-9c] a decrease in the granular degeneration was observed as compared to diabetic control group rats. Periportal inflammation, hepatocytes degeneration was less as compared to diabetic control group rats liver, In addition, no congestion in the central vein was observed [Table/Fig-9a-c].
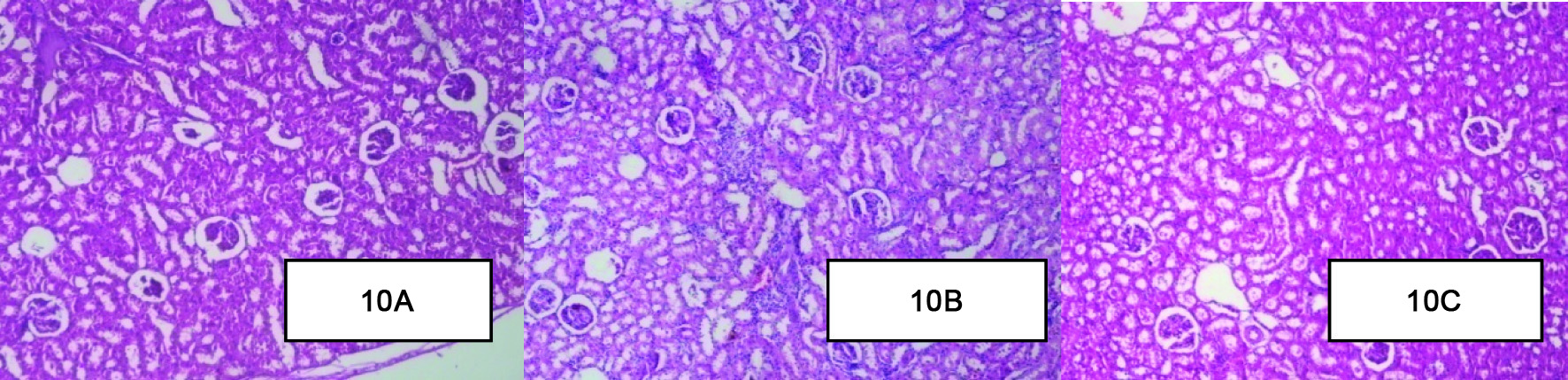
Effect of Berberine on Histopathology of the Kidney
Histopathology of healthy control group [Table/Fig-10a] kidney showed normal structure of the kidney. There was absence of congestion of glomerular blood vessels, tubular necrosis, inflammation and cloudy degeneration. In contrast histological assessment of the diabetic control group [Table/Fig-10b] showed congestion of glomerular blood vessels, tubular necrosis, inflammation and cloudy degeneration as compared to healthy control group. Berberine treated group [Table/Fig-10c] kidney showed congestion of glomerular blood vessels, tubular necrosis, inflammation and cloudy degeneration but it was mild as compared to diabetic control groups of rats [Table/Fig-10a-c].
Effect of Berberine on Histopathology of the Myocardium . (A): The healthy control group rat heart revealed the non-infarcted architecture of the myocardium (B): The diabetic control group rats subjected to STZ-ISP injury demonstrated marked oedema, confluent areas of myonecrosis separation of myofibers, congested blood vessels and mild inflammation. (C): Berberine treatment group rats, occasional focal myofiber loss, necrosis oedema and inflammation was observed. However, the degree of oedema, inflammation and necrosis was less as compared to the diabetic control group. (H&E x10).
Effect of Berberine on Histopathology of the Pancreas. (A): The healthy control group of rats pancreas were characterized by an organized pattern and showed normal architecture of islets of langerhans. (B): The diabetic control group of rats pancreas damaged islets of langerhans and the atrophy of beta cells. (C): In berberine treated group few nuclear changes were observed through less compare to diabetic control group. (H&E x10).
Effect of Berberine on Histopathology of the Liver. (A): The liver of the healthy control group rats, shows normal architecture of central vein, peripheral vein and hepatocytes. (B): The diabetic control group showed degeneration and scattered necrotic cells. (C): The berberine treated group periportal inflammation, hepatocytes degeneration was less as compared to diabetic control group rats liver, In addition, no congestion in the central vein was observed. (H&E x10).
Effect of Berberine on Histopathology of the Kidney (A): Histopathology of healthy control group kidney showed normal structure of the kidney. (B): The diabetic control group showed congestion of glomerular blood vessels, tubular necrosis, inflammation and cloudy degeneration. (C): Berberine treated group kidney showed congestion of glomerular blood vessels, tubular necrosis, inflammation and cloudy degeneration but it was mild as compared to diabetic control groups of rats. (H&E x10).
Discussion
The marketed synthetic DPP-IV Inhibitor, Sitagliptan has been reported to cause unacceptable adverse effects like pancreatitis. In this scenario, research to identify newer DPP-IV inhibitors that favourably modify various cardiovascular and metabolic risk factors, but are without noxious adverse effects of the synthetic DDP-IV Inhibitors, are warranted. Therefore, development of new class of natural DPP-IV Inhibitors like Berberine that work in concert with body’s own defense mechanism, having fewer side effects is the need of the hour. The present investigation was undertaken with objectives to evaluate and explore the cardio protective effects of berberine, known to possess DPP-IV Inhibitory activity, in the setting of diabetes mellitus. These findings may represent a novel adjunctive therapy to address the cardiovascular complications of diabetes.
Al-masri et al., demonstrated that commercially available pure form of berberine is an effective DPP-IV inhibitor [9]. Apart from the DPP-IV inhibition, it also shows other antidiabetic properties like insulin mimetic, reduction of insulin resistance, glycolysis promotion and enhancing the GLP-I release. The present study confirmed the hypoglycaemic effects of berberine (100 mg/kg). A significant decrease in the blood glucose and HbA1c levels in berberine treated group rats as compared to diabetic control group was observed. Observed hypoglycaemic effects of berberine are supported by the results of previous invitro [12,13] and invivo studies [14]. The study by Zhang et al., showed berberine significantly reduced the blood glucose and HbA1c in type 2 diabetic patients [15].
CPK-MB, an enzyme found primarily in the myocardium is used to evaluate the existence and extent of myocyte injury. It is the WHO that recognized gold standard indicative of myocardial damage [16]. When myocardial cells are damaged due to deficient oxygen supply or glucose, the integrity of cell membrane gets disturbed and it might become more porous that results in the leakage of these enzymes. Berberine (100 mg/kg) treated group showed decrease CPK-MB enzyme in treated rats as compared to diabetic control group rats. The study by Tianzhu Zhang et al., supported present study that Berberine (30 mg/kg, 60 mg/kg) reduced heart rate and ST-segment elevation, and decreased CK-MB and LDH enzyme in a dose-dependent fashion [17].
There are independent studies evaluating the antidiabetic and cardioprotective effects of berberine in either experimental models of diabetes or myocardial injury [17,18]. However, the present study is an effort to extrapolate these findings in a unique set up challenged with diabetes co-existing with myocardial injury. Diabetes was induced with streptozotocin and subsequently the diabetic rats were administered Isoproterenol (ISP), known to induce myocardial necrosis.
Isoproterenol, a synthetic catecholamine and beta adrenergic agonist, has been found to cause severe oxidative stress in the myocardium resulting in infarct-like necrosis of the heart muscles. Isoproterenol-induced myocardial necrosis is the most authenticated model of myocardial injury in rats [19]. However, there are studies which demonstrated the lack of cardiotoxic effect of Isoproterenol in Streptozotocin diabetic rats [20,21]. In contrast, the present study demonstrated that isoproterenol does induce myocardial necrosis. The acute myocardial injury induced by isoproterenol was confirmed by loss of integrity of myocardial membrane on histological examination. Histopathological examination revealed greater degree of myocardial, liver, kidney and pancreas cells oedema, inflammation, necrosis and degeneration of cells in diabetes control rats challenged with isoproterenol as compared to healthy control. It may be postulated that the dose of isoproterenol used in previous study (ranged from 0.008 to 30 mg/kg of body weight) may be insufficient to induce myocardial injury as compared to 85 mg/ kg dose used in the present study. Also, most of these studies have been conducted in the invitro setting of myocardial injury [22]. The results of Agrawal YO et al., concurs with these findings [11]. Thus, the present study provides evidence that this STZ-ISP model can be used for studying diabetes co-existing with myocardial injury and perhaps is a good model to screen antidiabetic drugs with cardioprotective potential.
Berberine demonstrated significant cardioprotective effects as indicated by myocardial CPK-MB levels in the setting of diabetes mellitus. Histopathological assessment of the myocardium confirmed the cardioprotective effects of Berberine. The degree of myocardial necrosis, inflammation and oedema was significantly less in the diabetic control group as compared to berberine treated group.
In addition to explore the cardioprotective mechanisms of berberine, lipid markers were also studied. It is well known that lipids play an important role in cardiovascular disease, by modifying the composition, structure and stability of cell membranes. Altered lipid metabolism is considered to accelerate the development of atherosclerosis, a major risk factor in myocardial infarction. High levels of circulating cholesterol and its accumulation in heart tissue is well associated with cardiovascular damage [23]. In the present study, Berberine showed favourable effects on lipid profile by reducing total cholesterol, triacylglycerol, LDL levels and favourably modifying HDL levels as compared to diabetic control group rats. As dyslipidemia is one of the important components of diabetes and insulin resistance [24,25], the reductions in particular of triacylglycerols levels by berberine may be beneficial to increase insulin sensitivity and promote glucose utilization in the peripheral tissues [26], Similar hypolipidemic effects have been showed by Yanfeng Chen et al., and Esfandiar Heidarian et al., [27,28].
The present study also evaluated the safety of berberine on the vital organs: liver and kidney. The markers {liver function (SGPT), kidney function (Creatinine)} were assessed in addition to histopathological evaluation of the degree of injury. Berberine significantly reduced SGPT and creatinine levels in the berberine group rats as compared to diabetic control group rats. Yibin Feng et al., also confirmed the hepatoprotective effects of berberine (80,120,160 mg/kg) [8]. Catabolism of the protein and nucleic acids results in the formation of non-protein nitrogenous compounds, urea and creatinine. In diabetes mellitus, the amino acid breakdown in the liver results in an increased production of urea and creatinine. Treatment with berberine was found to significantly decrease the creatinine levels. The Study by ISR. Punitha et al., also supports present study that, the berberine significantly reduced the creatinine level in diabetic rats [18].
Thus, the present study demonstrated that berberine produced myocardial salvaging effects in the setting of diabetes challenged with ISP induced myocardial necrosis. The cardioprotection may be afforded through a unique property of anti-diabetic (Glucose, HbA1c) and metabolic (Lipid Profile) activity. In addition, Berberine did not produce any cytotoxic effects as confirmed by liver function (SGPT), kidney function (Creatinine), myocardial (CPK-MB) markers and hisopathological studies.
DPP-IV based therapeutics may represent novel anti-diabetic drug, the cardioprotective actions of which may translate into demonstrable therapeutic benefits in diabetes with co-existing cardiovascular diseases. However, further studied are needed to confirm these finding and delineate the molecular mechanisms of the beneficial effects of Berberine.
Limitation
The present study did not compared the efficacy of test drug with conventional standard anti-diabetic drug.
Conclusion
Berberine treatment resulted in significant cardioprotective and myocardial salvaging effects as evidenced in diabetic rats. Berberine attenuated the deleterious cardiovascular changes observed in diabetes.