Multiple Small Intestine Perforations after Organophosphorous Poisoning: A Case Report
Rubina Khullar Mahajan1, Sudha Jasmine Rajan2, John Victor Peter3, Mayur Keshav Suryawanshi4
1 Senior Resident, Medical Intensive Care Unit (ICU), Department of Critical Care Medicine, Christian Medical College, Vellore, Tamil Nadu, India.
2 Associate Professor, Department of Medicine Unit 3, Christian Medical College, Vellore, Tamil Nadu, India.
3 Professor, Medical ICU, Department of Critical Care Medicine, Christian Medical College, Vellore, Tamil Nadu, India.
4 Assistant Professor, Department of General Pathology, Christian Medical College, Vellore, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Rubina Khullar Mahajan, Senior Resident, Medical Intensive Care Unit, Division of Critical Care Medicine, Christian Medical College, Vellore-632004, Tamil Nadu, India.
E-mail: khullar.rubina@gmail.com
Organophosphate poisoning has significant gastrointestinal manifestations including vomiting, diarrhea, cramps and increased salivation. We report an uncommon gastrointestinal complication of multiple small intestinal perforations following organophosphorus poisoning. A 28-year old male presented after ingesting dichlorvos mixed with alcohol. Following the initial cholinergic symptoms, the patient developed severe shock with fever, attributed to aspiration pneumonia. Despite appropriate antibiotics, shock was persistent. Over the next 24-hours, he developed abdominal distension, loose stools and high nasogastric aspirates. Computed tomography showed pneumoperitonium. Exploratory laparotomy revealed six perforations in the jejunum and ileum. The involved portion of the bowel was resected and re-anastomosed, following which only 80-cm of small bowel was left. Postoperatively, shock resolved over 72-hours. However, over the next few days, patient developed features of anastomotic leak. Since only a small portion of the small bowel was preserved, a conservative approach was adopted. He deteriorated further and finally succumbed to the illness.
Dichlorvos, Gastrointestinal, Organophosphate, Perforation
Case Report
A 28-year-old barber, a chronic alcohol consumer with history of depression, was brought to the emergency department within five hours of consumption of dichlorvos mixed with alcohol (quantity unknown), after he had one episode of generalized tonic clonic seizure. On admission he was in post-ictal state with a Glasgow coma score (GCS) of 3/15 (E1V1M1), pin-point pupils, copious oral secretions and incontinence of urine and faeces. His blood pressure was 150/80 mm Hg, pulse rate was 70/minute and oxygen saturation was 80% on room air. Arterial blood gas showed mixed metabolic and respiratory acidosis (pH 7.06, partial pressure of carbon dioxide (pCO2) 43, serum bicarbonate (HCO3) 11.6). Bilateral wheeze was heard on auscultation of the chest. Abdomen was soft and non-distended. He was intubated due to extensive secretions and atropine was administered in escalating doses (total dose 24 mg) to achieve atropinisation, followed by infusion at 2 mg/h. He also underwent gastric lavage and decontamination with activated charcoal. Serum pseudocholinesterase level was 42 U/l (reference range 3000-8000 U/l). He worsened rapidly in the emergency department, developed hypotension requiring two vasopressors and hence was shifted to the intensive care unit (ICU).
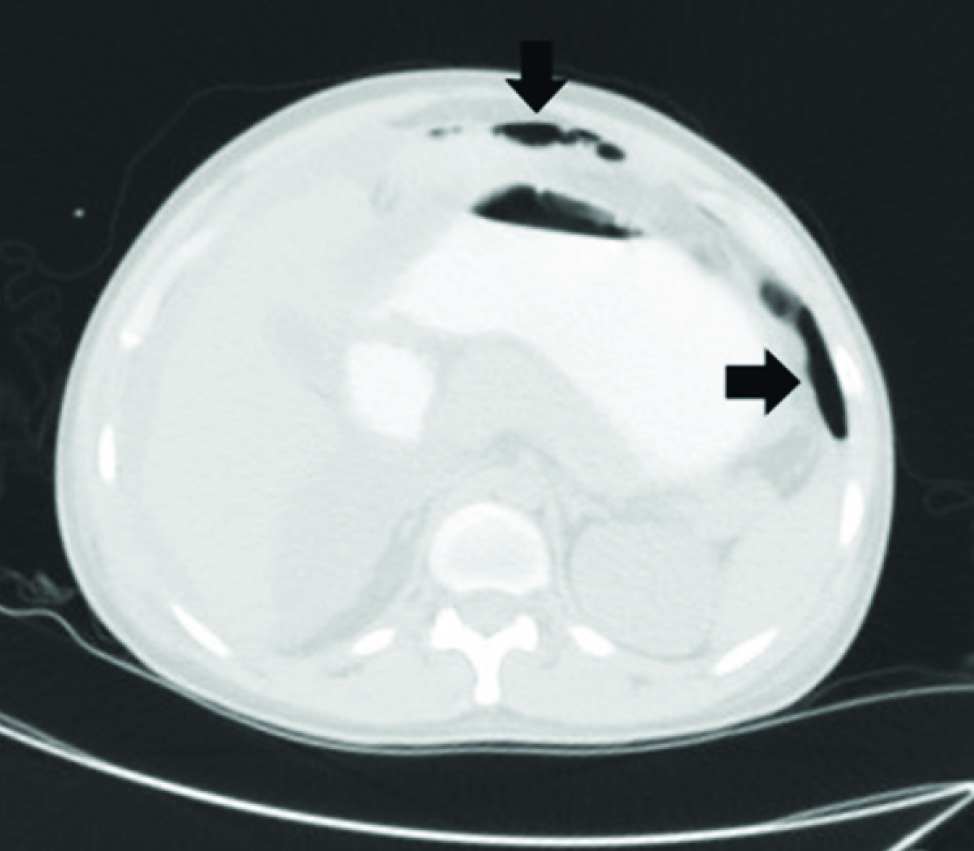
Following admission to ICU, he was found to have persistent high grade fever and refractory shock. Fever was initially attributed to aspiration pneumonia and he was started on Inj. Pipericillin/Tazobactam (4.5 grams intravenously every 8 hourly). Over the next 24-hours, the patient was noticed to have progressively worsening abdominal distension, loose stools and high nasogastric aspirates. There was persistent fever, hypotension requiring high dose vasopressor support and he continued to deteriorate with the development of renal failure. Chest and abdominal radiographs did not reveal air under the diaphragm. Haemodynamic instability precluded an immediate abdominal computed tomography (CT). Antibiotics were upgraded to Inj. Meropenem(1 gram intravenously every 8 hourly) and metronidazole (500 milligramsintravenously every 8 hourly) as an abdominal cause for sepsis was considered. Serum amylase 783 U/L (reference range 28-100U/L) and lipase 2474 U/L (reference range 13-60 U/L) were elevated. When he was relatively more stable, a CT of the abdomen was performed and this showed dilated small bowel loops and pneumoperitonium [Table/Fig-1]. Hollow viscus perforation was suspected and he underwent an emergency exploratory laparotomy. This revealed clumped small bowel loops covered with pus flakes and multiple inter-loop abscesses. There were six perforations in the small bowel (jejunal and ileal) and 2 litres of feculent peritoneal fluid with a peculiar odour. The most proximal perforation was 60cm from the duodenum and the most distal perforation was 20cm from the ileo-caecal valve. The involved portion of the bowel was resected and re-anastomosed. Following this, only about 80cm of the small bowel was remaining post resection and anastomosis. The intervening bowel was resected and histopathology of the resected specimen showed transmural haemorrhagic necrosis suggestive of perforation, patchy areas of ulceration lined by acute inflammatory exudates with oedema and congestion [Table/Fig-2]. The patient clinically improved after surgery and vasopressors were weaned within 72-hours. However, the abdominal drains continued to drain bile stained fluid and an anastomotic leak was suspected (as the bowel was very edematous at the time of surgery, probably due to hypoalbuminaemia). In view of the extent of small bowel involvement and a very small portion of small bowel left, conservative management was advised by the surgeons. Over the next few days, the patient deteriorated and finally succumbed to the illness on the eighth postoperative day.
CT image of abdomen with pneumoperitonium (arrows)
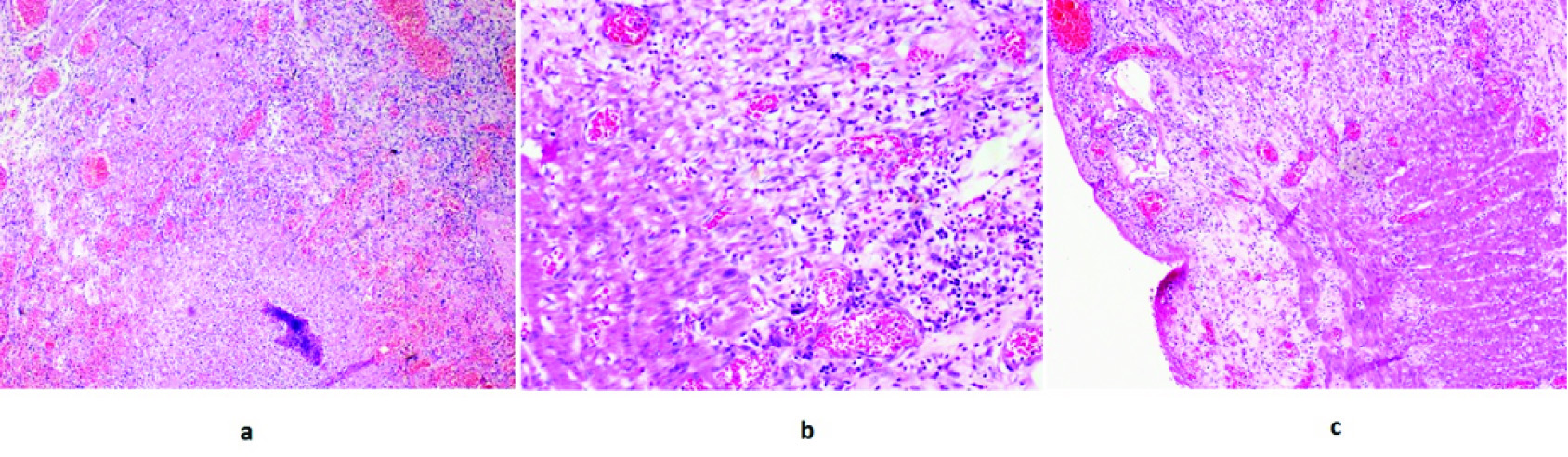
(a) H/E(10X): Shows discontinuity in the wall of small bowel with transmural haemorrhagic necrosis containing neutrophils, eosinophils, plasma cells and occasional histiocytes consistent with perforation. (b) H/E(40X): Shows muscularis layer with transmural haemorrhage, necrosis and moderate infiltrates of neutrophils, eosinophils, lymphocytes and plasma cells, consistent with perforation. (c) H/E(10X): Shows wall of small bowel with extensive ulceration and gracile haemorrhagic necrosis consistent with perforation.
Discussion
Organophosphorous poisoning is typically associated with significant gastrointestinal manifestations that include vomiting, diarrhea, cramps and increased salivation [1]. Other gastrointestinal complications like mucosal erosions [2], gastric ulcers with perforation and peritonitis [3], duodenal ulcers [3], lower gastrointestinal haemorrhage [4] and pancreatitis [5] have been reported in literature. Paralytic ileus may also occur as a consequence of atropine therapy [6]. We report a rare complication of multiple jejunal and ileal perforations following the consumption of dichlorvos.
Dichlorvos, a dimethyl organophosphorous compound, has been classified by World Health Organization (WHO) as Class I B (highly hazardous) pesticide [7]. It is toxic on inhalation, dermal absorption and ingestion. It acts by competitive inhibition of pseudocholinesterase and acetylcholinesterase enzymes [7]. It produces rapid onset of symptoms as it does not need to be metabolized to an active form. Effects from acute exposure include muscarinic, nicotinic and central effects [7]. A few uncommon presentations with gastrointestinal manifestations have been reported in literature [2–5].
On admission to the ICU, the patient was in shock. The possibilities considered were hypovolaemia (secondary to excessive salivation, defecation and urination), vasoplegia as a result of peripheral vasodilatation [8] and sepsis (aspiration pneumonitis), cardiogenic due to myocardial injury [1] or mixed forms of shock involving multiple mechanisms listed above. Since the shock persisted despite fluid resuscitation and resolved after source control (peritonitis), the shock was attributed predominantly to intra-abdominal sepsis.
Several differential diagnosis were considered in the evaluation of abdominal distension and included paralytic ileus due to metabolic causes (hypokalaemia secondary to high nasogastric aspirates) or atropine [6], pancreatitis [5] and mesenteric ischemia (in view of persistent metabolic acidosis and hyperlactatemia). However, CT of the abdomen showed features of bowel perforation.
The peculiar odour experienced by the surgeons during laparotomy in the absence of other perpetrators of gastrointestinal perforation makes the organophosphate compound the likely cause of bowel perforation in this patient. Organophosphorus poisoning is known to cause vascular endothelial dysfunction and the bowel perforation could have been due to microvascular thrombosis leading to bowel wall ischemia [4]. Organophosphates have also been directly implicated in causing intestinal ischemia and enteritis, which along with hypersalivation could also have contributed to hyperamylasaemia in our patient [9]. Other solvents present in the dichlorvos formulation could also have contributed to the mucosal erosions. The histopathology of the resected specimen of our patient showed acute inflammation and transmural haemorrhagic necrosis in the perforated segments, thus supporting the hypothesis that the perforations were due to gut ischaemia. Sparing of oesophagus and stomach can be possibly due to shorter contact time in oesophagus and a morevascular and thicker wall of the stomach. By the time atropine was administered to the patient, the toxin would probably have reached the small intestine and slowing of gut due to atropinisation would have further led to a longer contact time of the toxin with the small bowel mucosa, thus explaining the higher predilection of small bowel to the injury.
Conclusion
This case report highlights that in patients with organophosphate poisoning who present with acute abdomen, small bowel perforation must be considered as a differential in addition to toxin induced pancreatitis and atropine induced ileus as delay in intervention may be detrimental.
[1]. Peter JV, Sudarsan TI, Moran JL, Clinical features of organophosphate poisoning: A review of different classification systems and approachesIndian J Crit Care Med. Peer-Rev. Off. Publ Indian Soc Crit Care Med 2014 18:735-45. [Google Scholar]
[2]. Tashev TS, Markov D, [Stomach and duodenal lesions in patients with acute organophosphorus pesticide poisonings]V-treshni Boles 1991 30:61-65. [Google Scholar]
[3]. Tashev TS, [2 cases of severe gastroduodenal complications as a consequence of acute poisoning by organophosphorus pesticides]V-treshni Boles 1988 27:136-39. [Google Scholar]
[4]. Tanabe K, Ikezaki T, Takano A, Suzuki T, Kitazawa H, Terasaki T, A case report of organophosphorus pesticide poisoning resulted in delayed severe lower intestinal haemorrhageSci. Postprint [Internet] 2013 [cited 2015 Sep 2] :1Available from: http://www.spp-j.com/spp/1-1/spp.2013.12C0006/ [Google Scholar]
[5]. Hsiao CT, Yang CC, Deng JF, Bullard MJ, Liaw SJ, Acute pancreatitis following organophosphate intoxicationJ Toxicol Clin Toxicol 1996 34:343-47. [Google Scholar]
[6]. Beards SC, Kraus P, Lipman J, Paralytic ileus as a complication of atropine therapy following severe organophosphate poisoningAnaesthesia 1994 49:791-93. [Google Scholar]
[7]. DICHLORVOS - National Library of Medicine HSDB Database [Internet]. [cited 2015 Sep 3]; Available from: http://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+ hsdb:@term+@DOCNO+319 [Google Scholar]
[8]. Asari Y, Kamijyo Y, Soma K, Changes in the haemodynamic state of patients with acute lethal organophosphate poisoningVet Hum Toxicol 2004 46:5-9. [Google Scholar]
[9]. Yoshida S, Okada H, Nakano S, Shirai K, Yuhara T, Kojima H, Much caution does no harm! Organophosphate poisoning often causes pancreatitisJ. Intensive Care 2015 3:21 [Google Scholar]