Non Operative Approach to Isolated Traumatic Pancreatic Duct Disruption
Sheshang U Kamath1, Satish B Dharap2
1 Post Graduate Student, Lokmanya Tilak Municipal Medical College and General Hospital, Mumbai, Maharashtra India.
2 Professor, Department of General Surgery, Lokmanya Tilak Municipal Medical College and General Hospital, Mumbai, Maharashtra India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sheshang U Kamath, Lokmanya Tilak Municipal Medical College and General Hospital, Mumbai-400022, Maharashtra, India.
E-mail: sheshangkamath@gmail.com
Management of isolated traumatic pancreatic duct disruption remains challenging due to associated morbidity and mortality. Two children with isolated pancreatic ductal disruption were treated conservatively. Both developed a pseudocyst which resolved spontaneously due to the atrophy of the distal pancreas in a five-year-old girl while remained persistent and was treated by endoscopic cystogastrostomy in an eight-year-old boy. Non-operative management may be pursued in patients with pancreatic ductal injury in the hope of a pseudocyst formation which may spontaneously resolve or may be treated later with a minimally invasive procedure. However, the literature review precludes its practice as a standard due to high incidence of associated complications of non-operative management.
Blunt injury, Children, Conservative, Pancreas, Treatment
Case Reports
Case 1
A five-year-old girl presented with epigastric pain with the history of fall of a television set over her abdomen 4 days prior. On admission, patient had a pulse of 100 beats/minute, blood pressure of 96/60 mm of Hg and epigastric tenderness. Ultrasound of the whole abdomen was suggestive of bulky, hypoechoic pancreas with altered echotexture and peripancreatic fluid collection.
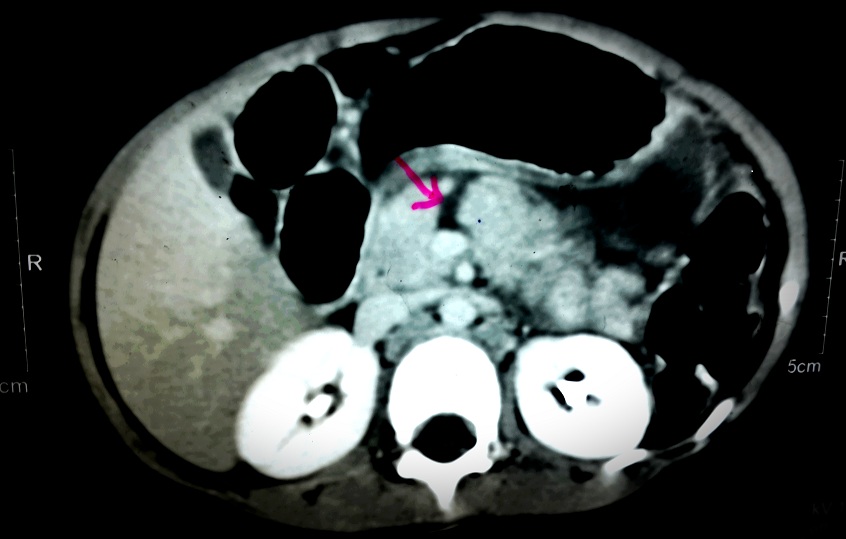
Contrast enhanced computerised tomography revealed complete transection of pancreas suspicious of ductal involvement [Table/Fig-1] which was later confirmed by MR cholangio-pancreatography [Table/Fig-2].
CT scan with arrowhead showing complete transection of pancreas with the major duct at the junction of body and neck with moderate amount of fluid in the lesser sac, anterior to the splenic vessels. Free fluid in abdomen and pelvis and bilateral mild pleural effusion also seen.
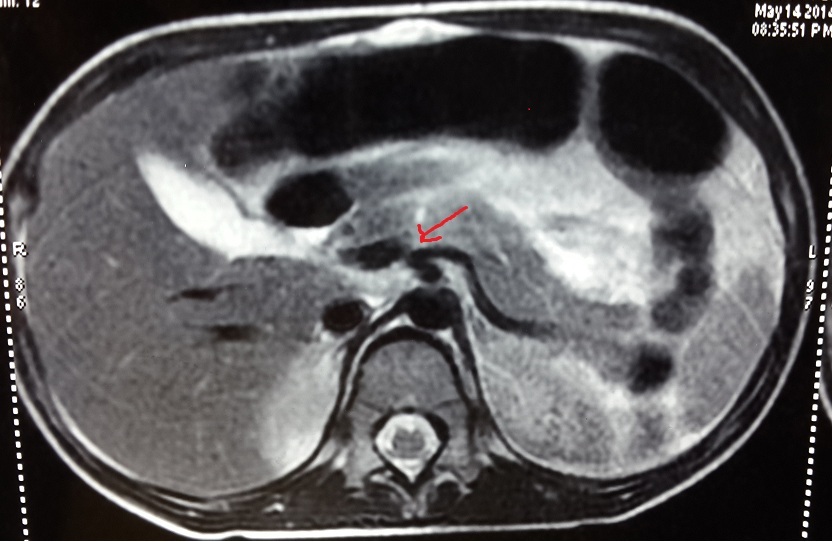
MRCP with arrowhead showing focal pancreatic laceration involving near complete parenchymal thickness with secondary pancreatitis and thin walled forming pseudocyst. Moderate ascites and bilateral mild pleural effusion also seen.
Patient was managed conservatively, kept nil by mouth on intravenous fluids, gastric aspiration and close monitoring. Preventive antibiotic therapy included injection cefotaxime and injection metronidazole along with proton pump inhibitors, analgesics and octreotide.
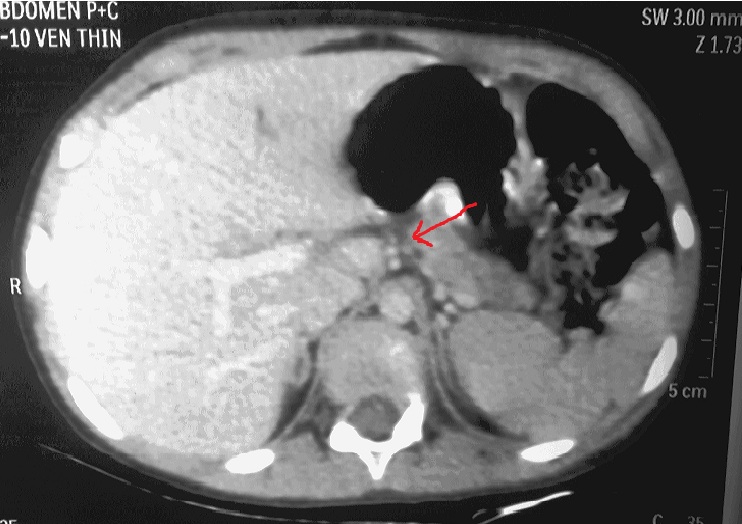
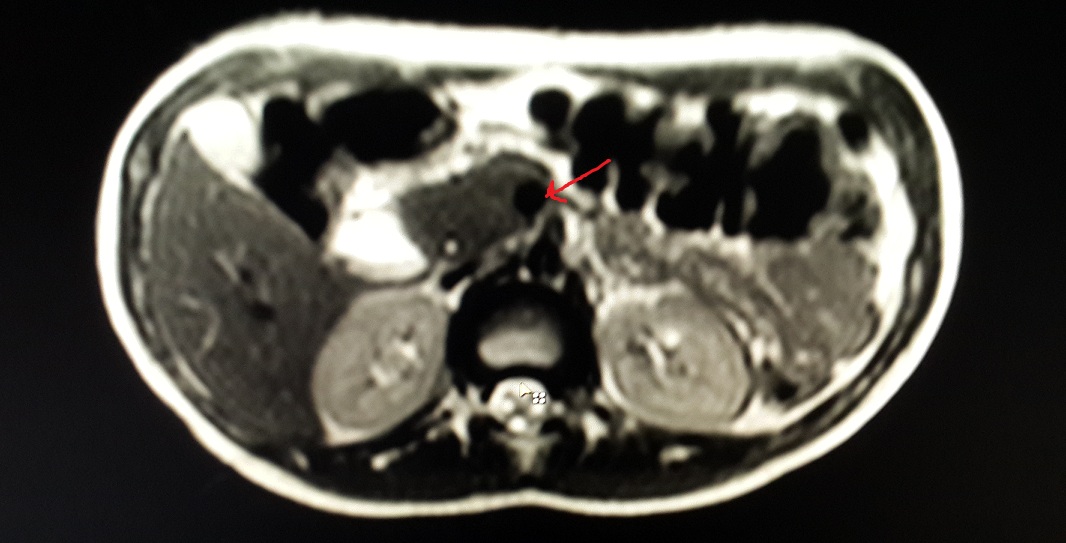
As the patient improved clinically and started accepting oral feeds, conservative management was continued and the pancreatic injury was further assessed using ultrasound which revealed well-organised fluid collection of size 11*7*6.9 cm by day-20 of admission, when she was discharged with a plan to perform elective cystogastrostomy upon the maturity of cyst wall. At 2 months follow up, ultrasound showed a well formed pseudocyst of size 6.*7*2.9*5.9 cm i.e. considerably smaller than earlier size. At 3 month follow up, the cyst was no longer visible on ultrasonography. Contrast enhanced computerised tomography then showed complete regression of the pseudocyst with parenchymal atrophy of the body and tail of pancreas with prominence of duct [Table/Fig-3]. The atrophy of the pancreas distal to the pancreatic laceration was confirmed by the MR scan [Table/Fig-4]. Thus despite the major ductal injury patient could be managed without surgery.
CT scan with arrowhead showing complete regression of the pseudocyst with mild parenchymal atrophy in body and tail of pancreas with prominence of duct.
MRCP showing complete parenchymal atrophy in body and tail of pancreas distal to the laceration. Arrowhead pointing at the laceration.
Case 2
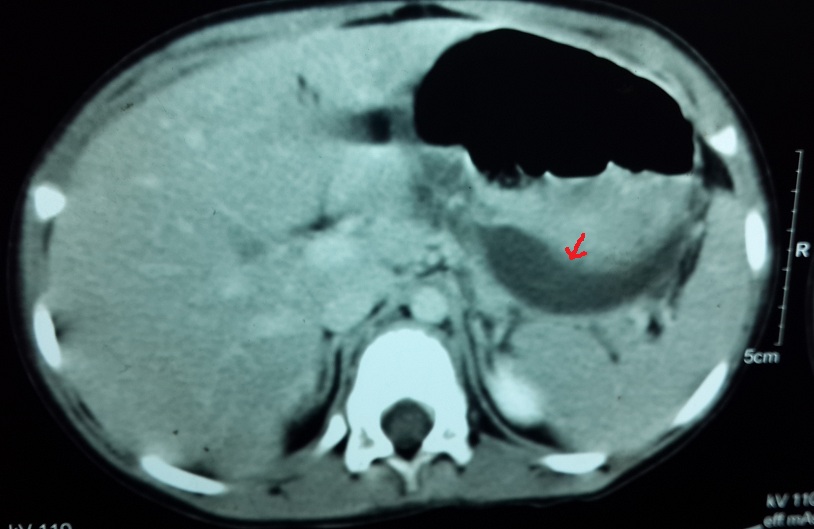
An eight-year-old boy presented with non bilious vomiting and abdominal pain, following a fall from a bicycle and handle bar injury to the abdomen one day prior. He was stable with normal blood investigations except serum amylase and lipase levels which were raised. The ultrasound of the abdomen showed a bulky pancreas with hypoechoic and inhomogeneous echotexture and a 4.3*1.5 cm sized well-defined heterogeneously hypoechoic collection in the left hypochondrium suggestive of a haematoma. Contrast enhanced computerised tomography was done on day 2 post incident which was suggestive of a laceration through the body of pancreas [Table/Fig-5]. With the conservative treatment (nil by mouth, nasogastric aspiration and intravenous fluids) his pain subsided and conservative treatment was continued.
CT shows laceration of body of pancreas extending from superior to inferior surface.(arrowhead) Splenic vessels appear grossly normal. Mild perisplenic fluid suggestive of hemoperitoneum.
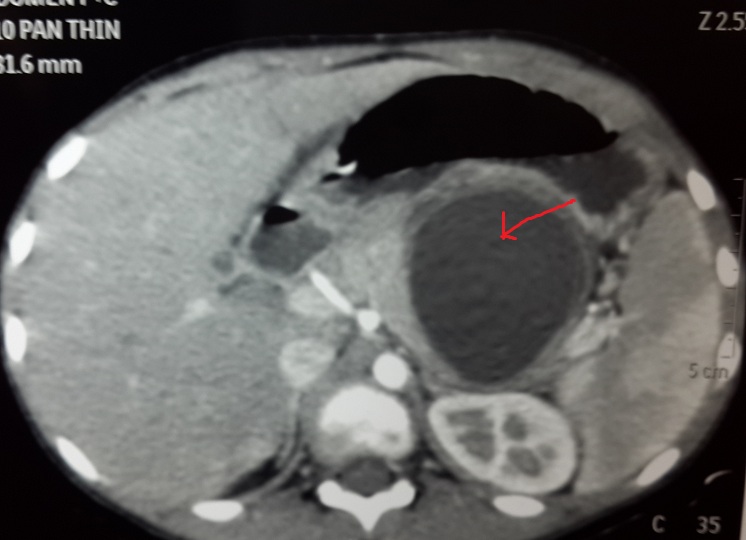
Patient clinically improved and oral feeds were started on day 7 of admission. However, repeat ultrasonography of the abdomen revealed 7.5*5.5*5 cm sized irregular collection in the lesser sac with multiple mobile internal echoes not seen separately from the body of the pancreas. This was further evaluated by a repeat CT scan which showed a well-organised fluid collection with suspicious communication with the main pancreatic duct [Table/Fig-6]. This was confirmed by MRCP [Table/Fig-7]. Patient was discharged as he was clinically well.
CT shows laceration of body of pancreas extending from superior to inferior surface and formation of large pseudocyst as shown by the arrowhead.
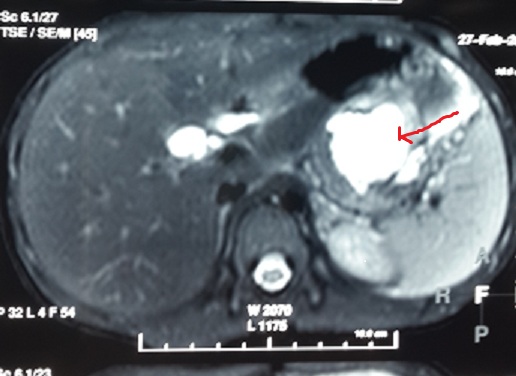
MRCP showing intra and peripancreatic fluid collection(arrowhead) secondary to traumatic laceration of distal part of body of pancreas with suspicious communication with the main pancreatic duct.
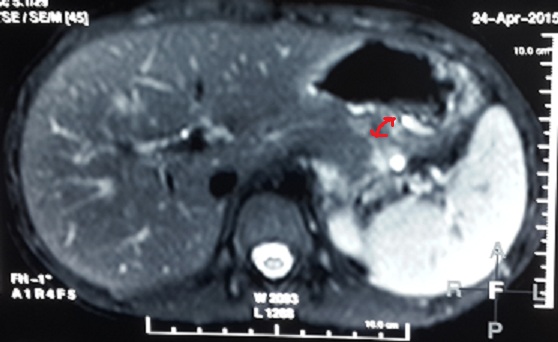
At one month follow-up he had developed a well defined epigastric lump and had complaint of discomfort. Endoscopic intervention was planned. Under endosonographic guidance and fluoroscopic control the pseudocyst was drained into the stomach using no. 10 F double pigtail stent. One month after the procedure MRCP showed no obvious fluid collection in the peripancreatic area [Table/Fig-8]. The stent was removed finally after 6 weeks the procedure.
MRCP showing no obvious fluid collection in the peripancreatic area with stent in situ (arrowhead).
Discussion
Despite the popularity of non-operative management of solid organ injuries, non-operative treatment of pancreatic ductal injury remains a controversy. Ductal status is an important predictor of outcome in pancreatic trauma and considered essential for establishing the basis for treatment decision. The literature reveals that the natural course of untreated pancreatic ductal disruption can be quite variable [1].
Blunt pancreatic trauma often results in leakage of enzymes and bleeding and may result in the systemic inflammatory response syndrome with manifestations similar to acute necrotising pancreatitis. Fleming WR et al., reported a 17% incidence of pancreatitis following trauma. Resolution occurred in two-thirds of their patients within several weeks [2].
Pancreatic abscess is another complication usually seen after delay in diagnosis of the pancreatic injury. The incidence has been reported at around 20% [3–5]. A pancreatic fistula is a common complication following pancreatic trauma. The incidence has been reported at around 20% in isolated pancreatic injury and around 35% following combined pancreaticoduodenal injuries [3–5]. Formation of pseudocyst should be regarded as a favourable course in the natural history of traumatic pancreatic ductal injury as internal drainage of a mature pseudocyst is a technically simple procedure with minimal morbidity [6,7].
Beres ALet al., in their recent article on pancreatic trauma concluded that primary non-operative management of high-grade pancreatic injuries is associated with a significant increase in complications and TPN dependency [8]. Early operative intervention should be pursued whenever feasible. Also, Ahmed N et al., state the overall mortality of pancreatic injury to be around 17% and a delay in diagnosis of more than 24 hours increased the mortality to 41% [9]. Recent literature mentions the overall mortality of pancreatic trauma as 20% [10].
Therefore, once ductal injury is ascertained by MRCP, expeditious pancreatic resection or pancreatico-jejunostomy has been advised to reduce morbidity and the length of hospital stay, particularly for injuries involving the tail and body [11,12]. If ductal injury can be ruled out, pancreatic injury can be safely treated non-operatively. When pancreatic ductal injury is diagnosed early, the window of opportunity for easy surgical management has been reported to exist in the first 48 hours for a stable isolated Grade III pancreatic injury [13]. Perhaps local inflammation makes surgery technically difficult after 48 hours.
Advances in technology such as endoscopic pancreatic ductal stenting and endoscopic ultrasound-guided drainage of pseudocysts have enhanced the scope of non-operative management of pancreatic injuries with ductal disruption also; as practised in the second case. Early ERCP guided pancreatic ductal stenting may be helpful to relieve leakage, but this is not well evidenced. Wood JH et al., suggested that therapeutic ERCP conferred no additional protection towards fluid collection in pancreatic trauma. (66% developed pancreatic fluid collections requiring drainage) [14].
In rare cases with pancreatic ductal disruption, the pseudocyst may spontaneously resolve and followed later atrophy of the pancreas distal to the pancreatic laceration, as could be observed in the first case. This has been previously documented by Wales PW et al., where in long term follow-up of patients with major pancreatic ductal lacerations, abdominal CT scans in 8 patients showed complete atrophy of the body and tail in 6 patients and 2 completely normal glands . After a median follow-up of 47 months, no patients had endocrine or exocrine dysfunction [7].
The principles of management of pancreatic trauma remain the same in both paediatric and adult population. However, most pancreatic injuries in children are isolated injuries unlike in adults who often have multiple abdominal injuries needing surgical intervention [15].
Pancreatic injury picked up on abdominal CT scan in stable patients with blunt abdominal trauma should be further evaluated by MRCP for ductal integrity. Non-operative management should be the strategy in all patients with intact pancreatic duct. Corrective surgery (resection or internal drainage) should be preferred within the first 48 hours of injury due to unpredictable course and high incidence of complications of non-operative management. Those presenting after a significant delay may be treated non-operatively in the hope of a pseudocyst formation or spontaneous distal atrophy. However, they need to be closely monitored for both local and systemic complications. While systemic complications may need critical care support; local complications may merit interventions like external drainage or surgical resection. Once the pseudocyst matures surgical or endoscopic internal drainage may be planned.
Conclusion
The complexity in the management of trauma to pancreas lies in its local and systemic complications. Therefore, surgical management remains the answer for trauma with less than 48 hours but delay would suggest conservative approach.
[1]. Subramanian A, Dente CJ, Feliciano DV, The management of pancreatic trauma in the modern eraSurg Clin North Am 2007 87:1515-32. [Google Scholar]
[2]. Fleming WR, Collier NA, Banting SW, Pancreatic trauma: Universities of Melbourne HPB groupAust NZJ Surg 1999 69:357-62. [Google Scholar]
[3]. Patton JH, Lyden SP, Croce MA, Pancreatic trauma: a simplified management guidlineJ Trauma 1997 43:234-41. [Google Scholar]
[4]. Flynn WJ, Cryer HG, Richardson JD, Reappraisal of pancreatic and duodenal injury. Management based on injury severityArch Surg 1990 125:1539-41. [Google Scholar]
[5]. Balasegaram M, Surgical management of pancreatic traumaCurr Probl Surg 1979 16:1-59. [Google Scholar]
[6]. Jobst MA, Canty TG, Lynch FP, Management of pancreatic injury in pediatric blunt abdominal traumaJ Pediatr Surg 1999 34:818-23. [Google Scholar]
[7]. Wales PW, Shuckett B, Kim PC, Long-term outcome after nonoperative management of complete traumatic pancreatic transection in childrenJ Pediatr Surg 2001 36:823-27. [Google Scholar]
[8]. Beres AL, Wales PW, Christison-Lagay ER, McClure ME, Fallat ME, Brindle ME, Non-operative management of high-grade pancreatic trauma: is it worth the wait?J Pediatr Surg 2013 48:1060-64. [Google Scholar]
[9]. Ahmed N, Vernick JJ, Pancreatic InjurySouth Med J 2009 102(12):1253-56. [Google Scholar]
[10]. Debi U, Pancreatic trauma: A concise reviewWorld J Gastroenterol [Internet] 2013 19(47):9003 [Google Scholar]
[11]. Buccimazza I, Thomson CR, Anderson F, Naidoo NM, Clarke DL, Isolated main pancreatic duct injuries spectrum and managementAm J Surg 2006 191:448-52. [Google Scholar]
[12]. Degiannis E, Glapa M, Loukogeorgakis SP, Smith MD, Management of pancreatic traumaInjury 2008 39:21-29. [Google Scholar]
[13]. Šnajdauf J, Rygl M, Kalousová J, Kucera A, Petru O, Pýcha K, Surgical management of major pancreatic injury in childrenEur J Pediatr Surg 2007 17(5):317-21. [Google Scholar]
[14]. Wood JH, Patrick DA, Bruny JL, Sauaia A, Moulton SL, Operative vs nonoperative management of blunt pancreatic trauma in childrenJ Pediatr Surg 2010 45:401-06. [Google Scholar]
[15]. Takishima T, Sugimoto K, Asari Y, Kikuno T, Hirata M, Kakita A, Characteristics of pancreatic injury in children: a comparison with such injury in adultsJ Pediatr Surg 1996 31(7):896-900. [Google Scholar]