Ileo-ileal Intussusception and Bowel Obstruction Caused by Plasmablastic Lymphoma of Small Bowel- A Rare Entity in Rare Location
Aditya Atul Kulkarni1, Sanjiv S. Thakur2
1 Senior Resident, Department of General Surgery, B. J. Government Medical College, Pune, India.
2 Professor and Head, Department of General Surgery, B. J. Government Medical College, Pune, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Aditya Atul Kulkarni, C/O Dr. Mankikar, 101, DSK Rohan, Near Model Colony Post Office, Model Colony, Shivajinagar, Pune- 411016, India.
E-mail: draadityakulkarni@gmail.com
Intussusception of small bowel is considered a rare cause of bowel obstruction in adults accounting for only about 1% of bowel obstruction in adults. Intussusception in adults is uncommon with 95% cases of intussusceptions occurring in children. Adult intussusception from small intestinal lymphoma is also rare with only 36 cases reported in the literature between 2000 and 2011. Plasmablastic lymphoma (PBL) is an aggressive lymphoid neoplasm usually seen in the oral cavity in the clinical setting of human immunodeficiency virus (HIV) infection. Plasmablastic lymphoma of the small intestine is extremely rare. Here, we report a case of plasmablastic lymphoma of small bowel with ileoileal intussusception in an HIV-negative immunocompetent male patient.
Abdominal Pain, B-Cell, Epstein-Barr Virus Infections, Paraproteinemias, Plasmacytoma
Case Report
A 42-year-old male patient presented with complaints of severe abdominal pain and vomiting since three days. Pain was periumbilical, colicky in nature and associated with numerous episodes of bilious vomiting. Patient had not passed stools since past three days. He reported anorexia and weight loss of 14 kg over three months. There was no past history of co-morbid medical or surgical illness.
On clinical examination, he was pale, tachycardic and hypotensive. Abdominal examination revealed generalized abdominal distension and Diffuse tenderness. There was no guarding, rigidity or peritoneal signs. Per-rectal examination revealed an empty rectum. On lab investigations, liver and kidney function tests were within normal limits. There was anaemia and leukocytosis (Haemoglobin-8.5 gm/dl, WBC-16×109/L). An abdominal X-ray revealed distended loops of small bowel with air-fluid levels.
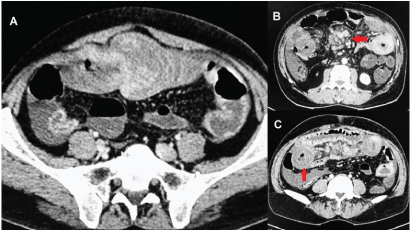
Provisional diagnosis of acute small bowel obstruction was made. CT scan of abdomen revealed ileo-ileal intussusception in umbilical region [Table/Fig-1a]. There was a large lymph nodal mass (5 x 4 x 4 cm) in the mesentery of small bowel [Table/Fig-1b] along with paraaortic lymphadenopathy. Bowel loops were grossly thickened and edematous (wall thickness 2.5- 3 cm) [Table/Fig-1c].
Computed tomography of abdomen: a) Computed tomography revealed an ileo-ileal intussusception in umbilical region; b) along with large lymph nodal mass in the mesentery (arrow); and c) grossly thickened small bowel loops (arrow).
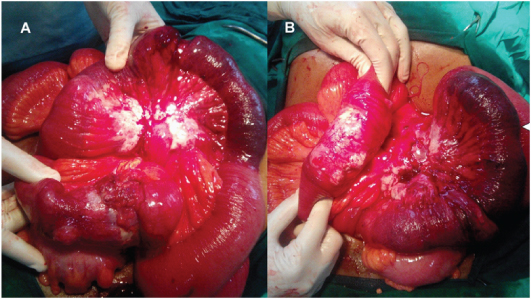
The patient was explored by midline laparotomy. There was a short segment of ileoileal intussusception seen along with a large, hard mesenteric mass with enlarged mesenteric nodes [Table/Fig-2a,b]. The involved segment along with the mesentery containing the mass of lymph nodes was resected with 5 cm margins and end to end anastomosis with healthy small bowel was performed. Postoperatively, the patient made an uneventful recovery.
Findings at laparotomy. Findings at laparotomy included short segment ileo-ileal intussusception and thickened bowel loops with large mesenteric mass.
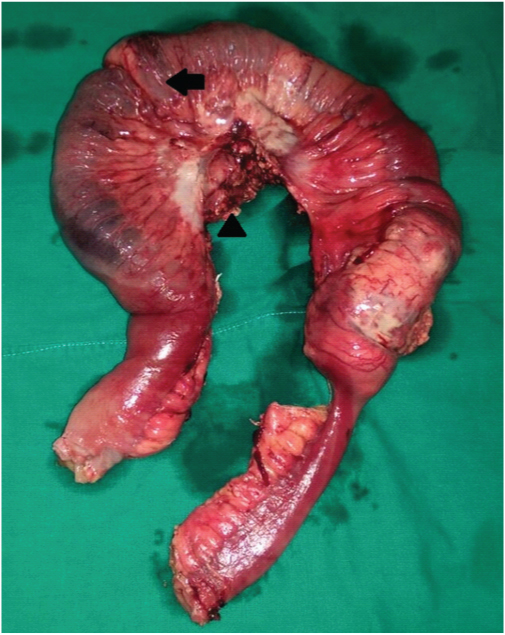
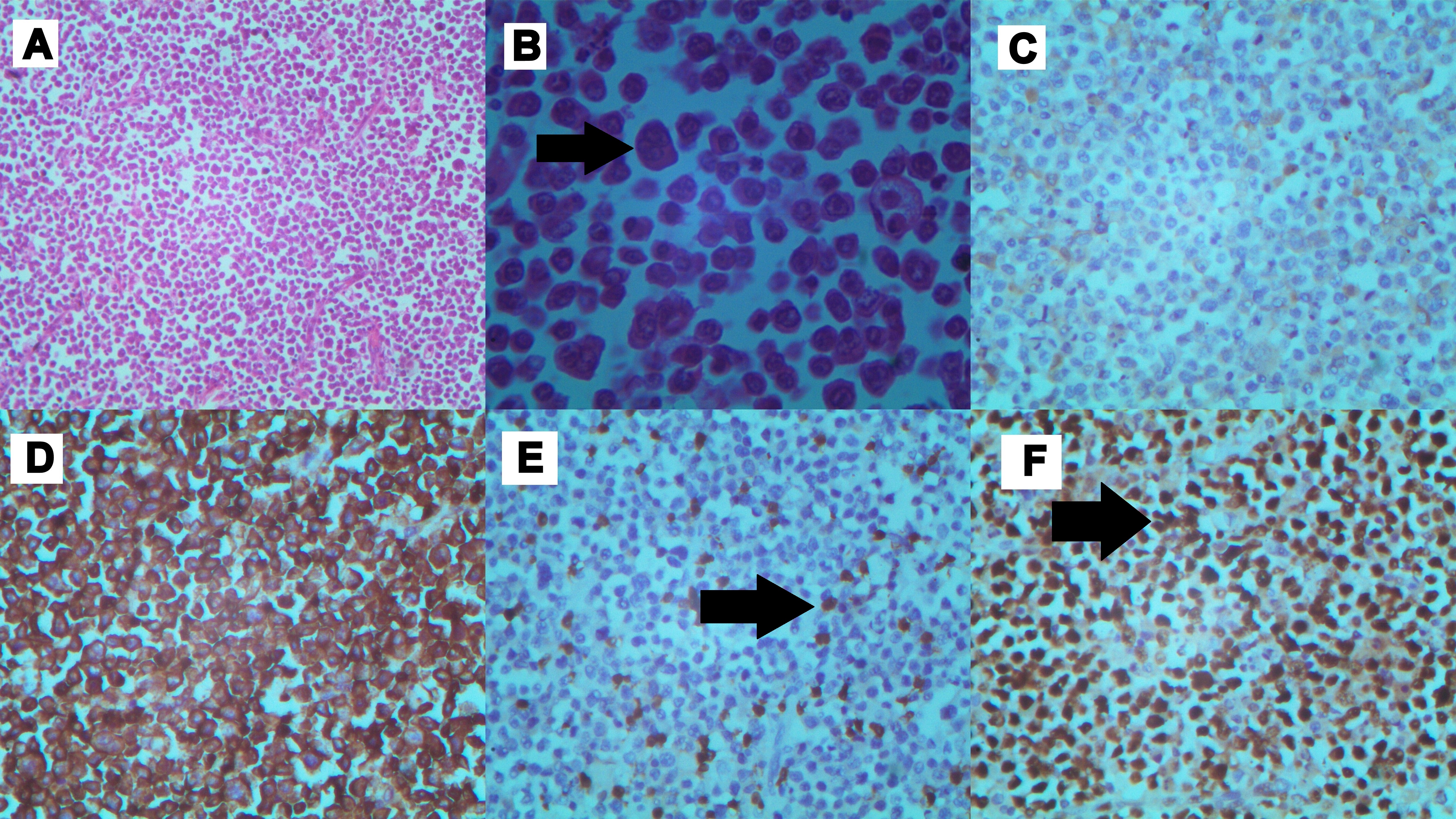
Surgical specimen comprised of 25 cm long ileal segment with its mesentery and nodal mass [Table/Fig-3]. Cut sections of the specimen revealed thickened bowel wall with mucosal ulcerations and haemorrhages. Histopathology revealed proliferation of medium to large lymphoid cells with eccentric nuclei with frequent mitoses and necrosis [Table/Fig-4a,b]. On immunostaining the cells were negative for CD20 [Table/Fig-4c], but strongly reactive to CD38 and CD138 [Table/Fig-4d] with scattered positivity for CD3 [Table/Fig-4e]. Proliferation index by Ki-67 immunohistochemistry was close to 90% [Table/Fig-4f]. Bone marrow biopsy was normal. PET- CT and CT thorax showed multiple enhancing lymph nodes in superior mediastinum. Serum protein electrophoresis and urinary protein excretion were normal. Serological tests for Ebstein-Barr virus (EBV) were positive. He tested negative for human immunodeficiency virus (HIV). Diagnosis of high-grade plasmablastic lymphoma (PBL) was made on basis of presentation, immunophenotype and morphology. The final staging as per modified Ann-Arbor classification was stage IIIE.
Resected surgical specimen. The specimen included 25 cm of ileum with intussusception (arrow) and lymph node mass (arrowhead).
Microscopic findings: a) Low power view showing “starry sky” appearance (H&E, magnification x10); b) High power view showed sheets of large atypical lymphoid cells (denoted by black arrows) with big round to oval nuclei, dense chromatin, prominent nucleoli and abundant cytoplasm suggestive of plasmablastic differentiation. (H&E, magnification x400); c) Cells were negative for CD20 (immunoperoxidase, magnification x40); d) Cells staining positively for CD138 (immunoperoxidase, magnification x40); e) Neoplastic cells neagative for CD3 with scattered T-cells positively staining. T- cells denoted by black arrows (immunoperoxidase, magnification x40); f) High proliferation index highlighted by Ki67 immunostain denoted by black arrows (approximately 90%) (Immunoperoxidase, Magnification × 40).
The patient was started on a Chemotherapy regimen of cyclophosphamide, hydroxydaunorubicin, oncovin and prednisone (CHOP) for a total of six cycles. There was good initial response, however the disease relapsed 9 months after initial diagnosis and the patient developed a large mesenteric mass with massive ascites. He also developed bilateral pleural effusion and tracheal compression with a large mediastinal mass. He was readmitted to intensive care with severe cachexia and succumbed after one week due to multi-organ failure.
Discussion
Intussusception in adults accounts for only around 5 % of all intussusceptions which accounts for only about 1% to 5% of all cases of bowel obstruction in adults [1,2]. A malignant etiology accounts for 30-70% of intussusceptions in adults [3]. In small bowel intussusceptions common causes are metastatic tumours, adenocarcinoma, gastrointestinal stromal tumours, lymphoma, and carcinoid tumours [3]. Out of these, lymphoma of small bowel as a cause of intussusception is quite rare. A review of English language literature revealed only 36 cases of intussusception due to lymphoma from 2000 to 2011 [3]. Another review of intestinal lymphoma cases reported that intussusception occurred in 46% and emergency surgery was required in upto 64% of patients [4].
Plasmablastic lymphoma was first described by Delecluse HJ [5]. It has now been recognized as a distinct entity by World Health Organization (WHO) classification of lymphoproliferative disorders. PBL have been strongly associated with immunodeficiency, particularly in patients with the HIV infection. There have been few reports of PBL in HIV negative patients, with majority in immunosuppressed individuals [6]. Notable feature of PBL is its strong predilection for oral cavity in majority of patients. Extraoral sites of PBL have been reported and incidence of these appears to be rising. Gastrointestinal tract accounts for only around 14% of extraoral primary sites. The incidence is quite low. In a search of PubMed for cases of gastrointestinal PBL in one study, only 18 cases were found in a period from 1998 to 2013 [7]. A more recent review from 2015 focusing on cases of PBL on PubMed/Medline, EMBASE, and Google Scholar published from 1997 to October 2014 found 83 cases involving gastrointestinal tract out of which HIV negative patients accounted for around 20% [8].
Clinically, PBL usually develops in middle-aged adults and is more prevalent among men. The most common symptoms at presentation are abdominal pain, weight loss, anorexia, and melena. Epstein Barr Virus (EBV) seems to play an important role in oncogenesis of HIV-associated PBL [9]. However, studies suggest that this virus may not be a participant in causing PBL in all cases and especially HIV negative patients [10]. Our patient tested positive for EBV RNA but was not HIV positive or immunosuppressed. PBL is frequently misdiagnosed as poorly differentiated carcinoma or sarcoma as it does not express common lymphoid markers. It also needs to be differentiated from similar lymphoid proliferative disorders like anaplastic large cell lymphoma, anaplastic plasmacytoma, extramedullary plasmablastic myeloma and diffuse large B-cell lymphoma [8,11]. This is done primarily on basis of light microscopic and immunostaining findings.
In this case, there was evidence of medium to large lymphoid cells with plasmacytoid differentiation. On immunostaining, the tumour cells were strongly positive for CD38 and CD138 and were negative for Bcl-6, CD20, CD30 and CD45. Hence considering all the possibilities, a final diagnosis of PBL was made in present case. There is currently no consensus on treatment of PBL. Substantial proportion of patients may present in emergency with an acute abdomen. For these patients with evidence of obstruction or perforation, early surgical management is advocated. Adequate oncologic resection with clear margins and wide excision of lymph node containing mesentery is a must. Chemotherapy regimens that have been used with partial or complete response include CHOP (cyclophosphamide, hydroxydaunorubicin, oncovin and prednisone), infusional EPOCH (Etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin), hyperCVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate and cytarabine), and CODOX-M/IVAC (Cyclophosphamide, Vincristine, Doxorubicin, Methotrexate, Ifosfamide, Mesna, Etoposide, Cytarabine) [6,7,12]. For HIV positive patients, antiretroviral therapy and monitoring of HIV viral titers and CD4 count also play a critical role in the treatment. There has been some success in adding bortezomib to Chemotherapy regimen or having patients undergo hematopoietic stem cell transplantation if remission is achieved [13]. More aggressive Chemotherapy regimens have not shown to produce a statistically significant improvement in outcome [12].
PBL is a highly aggressive malignancy, with majority of patients succumbing to the disease in the first year after diagnosis [6,7,14]. Interestingly, HIV-positive patients have a better response to Chemotherapy and longer survival time than HIV-negative patients [10]. Plasmablastic cytology, loss of CD20 and high Ki-67 index is associated with worse prognosis [15]. Generally, the disease at extra-oral sites tends to be disseminated at diagnosis [15].
Conclusion
We report a rare case of plasmablastic lymphoma involving the small intestine in a HIV-negative immunocompetent patient, who presented with small bowel obstruction due to ileoileal intussuception. Based on the findings in present case, PBL must be considered as a cause of intussusception in HIV positive as well as HIV negative patients. PBL is easy to misdiagnose it as a poorly differentiated carcinoma/ sarcoma. Clinicians and pathologists must be vigilant for correct diagnosis and early management in order to achieve a good outcome in patients suffering from this rare and aggressive malignancy.
[1]. Barussaud M, Regenet N, Briennon X, de Kerviler B, Pessaux P, Kohneh-Sharhi N, Clinical spectrum and surgical approach of adult intussusceptions: a multicentric studyInt J Colorectal Dis 2005 21(8):834-39. [Google Scholar]
[2]. Wang N, Cui XY, Liu Y, Long J, Xu YH, Guo RX, Adult intussusception: a retrospective review of 41 casesWorld J Gastroenterol 2009 15:3303-08. [Google Scholar]
[3]. Akbulut S, Unusual cause of adult intussusception: diffuse large B-cell non-Hodgkin’s lymphoma: a case report and reviewEur Rev Med Pharmacol Sci 2012 16:1938-46. [Google Scholar]
[4]. Abbott S, Nikolousis E, Badger I, Intestinal lymphoma—a review of the management of emergency presentations to the general surgeonInt J Colorectal Dis 2014 30(2):151-157. [Google Scholar]
[5]. Delecluse HJ, Anagnostopoulos I, Dallenbach F, Hummel M, Marafioti T, Schneider U, Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infectionBlood 1997 89:1413-20. [Google Scholar]
[6]. Castillo J, Reagan J, Plasmablastic Lymphoma: A Systematic ReviewSci World J 2011 11:687-96. [Google Scholar]
[7]. Luria L, Nguyen J, Zhou J, Jaglal M, Sokol L, Messina JL, Manifestations of gastrointestinal plasmablastic lymphoma: A case series with literature reviewWorld J Gastroenterol 2014 20(33):11894-903. [Google Scholar]
[8]. Castillo J, Bibas M, Miranda R, The biology and treatment of plasmablastic lymphomaBlood 2015 125(15):2323-30. [Google Scholar]
[9]. Grywalska E, Markowicz J, Grabarczyk P, Pasiarski M, Roliński J, Epstein-Barr virus-associated lymphoproliferative disordersPostepy Hig Med Dosw 2013 67:481-90. [Google Scholar]
[10]. Castillo J, Winer E, Stachurski D, Perez K, Jabbour M, Milani C, Clinical and pathological differences between human immunodeficiency virus-positive and human immunodeficiency virus-negative patients with plasmablastic lymphomaLeuk Lymphoma 2010 51(11):2047-53. [Google Scholar]
[11]. Bautista-Quach M, Ake C, Chen M, Wang J, Gastrointestinal lymphomas: Morphology, immunophenotype and molecular featuresJ Gastrointest Oncol 2012 3(3):209-25. [Google Scholar]
[12]. Castillo J, Plasmablastic lymphoma: Are more intensive regimens needed?Leuk Res 2011 35(12):1547-48. [Google Scholar]
[13]. Liu J, Zhang L, Ayala E, Field T, Ochoa-Bayona J, Perez L, Human immunodeficiency virus (HIV)-negative plasmablastic lymphoma: A single institutional experience and literature reviewLeuk Res 2011 35(12):1571-77. [Google Scholar]
[14]. Teruya-Feldstein J, Chiao E, Filippa DA, Lin O, Comenzo R, Coleman M, CD20-negative large-cell lymphoma with plasmablastic features: a clinically heterogenous spectrum in both HIV-positive and -negative patientsAnn Oncol 2004 15(11):1673-79. [Google Scholar]
[15]. Chuah KL, Ng SB, Poon L, Yap WM, Plasmablastic Lymphoma Affecting the Lung and Bone Marrow With CD10 Expression and t(8;14)(q24;q32) translocationInt J Surg Pathol 2008 17(2):163-66. [Google Scholar]