Natural T Regulatory Cells (n Treg) in the Peripheral Blood of Healthy Subjects and Subjects with Chronic Periodontitis – A Pilot Study
Ram Sabarish1, Suresh Ranga Rao2, Vamsi Lavu3
1 Senior Lecturer, Department of Periodontology, Faculty of Dental Sciences, Sri Ramachandra University, Chennai, India.
2 Professor and Head, Department of Periodontology, Faculty of Dental Sciences, Sri Ramachandra University, Chennai, India.
3 Associate Professor, Department of Periodontology, Faculty of Dental Sciences, Sri Ramachandra University, Chennai, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Suresh Ranga Rao, Professor and Head, Department of Periodontology, Faculty of Dental Sciences, Sri Ramachandra University, No.1, Ramachandra Nagar, Porur, Chennai – 600116, Tamilnadu, India. E-mail : chennaidentist@gmail.com
Introduction
The T cells play a central role in the aetiopathogenesis of periodontal disease. Natural T regulatory cells (nTreg) are the key stone immunoregulatory elements having an anergic phenotype and play an important role in the suppression of exaggerated immune responses thereby maintaining homeostasis. There are increasing evidences for the role of nTreg in the periodontal disease pathogenesis.
Aim
To identify the proportion of natural T regulatory cells in the peripheral blood of periodontally healthy subjects and subjects with chronic periodontitis.
Materials and Methods
A total of 15 subjects (7 with healthy gingiva and 8 with chronic periodontitis) were recruited for this pilot study. Baseline periodontal parameters were recorded and 5 ml of peripheral blood was collected. The samples from both the groups were analysed for the relative proportion of nTreg (identified by the expression CD45RB+CD4+CD25+FOXP3+) using flow cytometry.
Results
The mean percentages of the CD45RB+CD4+CD25+ cells expressing FOXP3 in control and chronic periodontitis group were found to be 14.75±5.04 and 43.13±11.17 respectively. The mean proportion of nTreg were compared between the control and chronic periodontitis sample using Mann-Whitney Test and was found to be statistically significant with (p<0.001).
Conclusion
A higher proportion of nTreg in the peripheral blood sample of chronic periodontitis subjects were observed as compared to that of healthy individuals.
CD25, FOXP3, Periodontal disease, Regulatory T lymphocytes
Introduction
Chronic Periodontitis is an inflammatory condition resulting from an interaction between the host’s immune system and the microorganisms in dental plaque [1]. Recently, periodontitis was categorized as a disease of dysbiosis, where a disruption of the host/microbial homeostasis is considered the key for disease initiation and progression [2,3]. This model proposes that multiple bacteria with differential roles and their cooperative combinations are required for the development of periodontal disease. In addition to the putative periodontal pathogens, other environmental and host-based factors may also be responsible for the disturbances in the host–microbe homeostasis. Once there is a perturbation of this homeostasis, accumulating evidence points to the fact that distinct patterns of immune response (proinflammatory, Th1 and Th17 cytokines) have the potential to increase inflammation and mediate osteoclastogenesis invivo, leading to periodontal tissue destruction. Most of the researches performed in this area have been focussing on the disease mechanism per se (i.e., disrupted host-microbe homeostasis) and only few works have been carried out to identify the factors responsible for maintaining the homeostasis [4]. The basis of host-microbial homeostasis depends upon the host’s ability to discriminate between harmful and harmless antigens. The type of immune response that ensures such homeostatic interaction is defined as immunologic tolerance [5]. Immunological tolerance is an active response that preserves physiological/functional tissue homeostasis while simultaneously retaining the capacity to respond to pathogenic antigens [6]. This tolerance response is achieved by a subset of immune cells called regulatory T cells (Tregs).
Tregs are subtype of CD4+Tcells, representing barely 5–10% of the peripheral T cell pool, which regulate other leukocyte function, avoid excessive immune activation and its pathological consequences [7]. Tregs are considered as “keystone immunoregulatory elements”, since they represent a low abundance leukocyte subset with a wide impact on the immune system [8]. Based on the origin, Tregs are of two types: Natural Treg cells (nTreg) that originate in the thymus and Inducible Treg cells (iTreg) that are generated in certain peripheral tissues in response to nonself (such as microbial) antigens [9].
Naturally occurring Treg cells have been found to be cluster of differentiation (CD) CD4+ and CD25+ [10]. The characterization of this T cell subset has been a challenge due to disparity in the reported molecular markers. Maggi E et al., stated that nTreg are phenotypically characterized by the presence of Glucocorticoid induced Tumor Necrosis Factor-Receptor (GITR), Cytotoxic T lymphocyte Antigen-4 (CTLA-4) [11]. But Nakajima et al., disagreed with this statement reporting that GITR and CTLA-4 are not reliable markers for the characterization of CD4+ CD25+T regulatory cells as they can also be expressed on other cell types [12]. The authors described forkhead box P3 (FOX P3) as a definitive marker for identifying nTreg since Fox p3 is the key transcription factor controlling T regulatory cell (Treg) development and function [13]. Induction of the FOXP3 gene in normal naïve T cells converts them to Treg-like cells with invivo and invitro suppressive function [13].
A pioneering work by Nakajima et al., demonstrated the presence of CD4, CD25, FOXP3 positive cells in healthy, gingivitis and periodontitis tissue samples with a higher proportion being observed in periodontitis tissues [12].
Aim
The objective of the present study was to identify the proportion of natural T regulatory cells in the peripheral blood of periodontally healthy subjects and subjects with chronic periodontitis.
Materials and Methods
Patient selection: An experimental pilot study was done involving a total of 15 subjects attending the out-patient clinic of the Department of Periodontology, Faculty of Dental Sciences, Sri Ramachandra University. The study was approved by the Institutional Ethics Committee (CSP/12/APR/22/77). Subjects were recruited for this study after obtaining their informed consent and were categorized into two groups based on specific inclusion and exclusion criteria as listed in [Table/Fig-1]. Group 1 comprised of 7 volunteers with clinically healthy gingiva and Group 2 comprised of 8 patients who were diagnosed to have generalized chronic periodontitis based on the clinical criteria stated in the 1999 report of the International Workshop for a Classification of Periodontal Diseases and Conditions [14]. The following clinical parameters were assessed: Oral Hygiene Index Simplified (OHI-S), Probing pocket depth and clinical attachment level were assessed at six sites per tooth using a University of North Carolina (UNC) 15 periodontal probe.
Criteria for subject selection.
| INCLUSION CRITERIA |
|---|
| Group 1 –Controls (Individuals with Healthy gingiva) | Group 2 – Test (Individuals with Chronic Periodontitis) |
|---|
| • The presence of probing depth (PD) <3 mm | • The presence of at least 10 natural teeth |
| • Absence of bleeding on probing (BOP) | • Generalized CP as evidenced by the presence of AL ≥1 mm in >30% of the sites examined |
| • No clinical attachment loss (AL) | • Abundant local factors |
| • No mobility or furcation involvement | • Radiographic evidence of bone loss |
| • No previous history of periodontal disease | |
| EXCLUSION CRITERIA FOR BOTH THE GROUPS |
| • Tobacco use in any form |
| • Subjects who had taken antibiotics in the past 6 months/ analgesics in the past 1 week |
| • Pregnant and lactating women |
| • Previous history of periodontal disease /periodontal therapy in 6 month prior to this study |
| • History of any other systemic disease/ medication for other systemic conditions |
Sample collection: A 5 ml of whole peripheral blood was collected from the antecubital vein in EDTA–coated vacutainer tubes (K2 EDTA) 5.4mg (BD vacutainer) under standard aseptic conditions. The peripheral blood was drawn prior to the start of Phase I therapy in healthy controls as well as chronic periodontitis subjects. The samples were immediately transferred to the lab for processing and flow cytometric analysis.
Sample Processing and Flow Cytometric Analysis: The peripheral blood mononuclear cells were separated from the collected blood sample by gradient density centrifugation using Ficoll Hypaque medium (2000 rpm × 20 mt). The cells thus separated were resuspended in 800 μl of Becton Dickinson (BD) pharmingen stain fetal bovine serum (FBS) buffer. The sample was then divided into two 400 μl suspensions: one half used for detecting the level of autofluorescence (unstained sample) and the other half of the suspension was subjected to cell surface staining with following fluorochrome conjugated monoclonal antibodies (Becton Dickinson, Heidelberg, Germany): anti-Human CD 45 RB (Phycoerythrin PE), anti-Human CD 4 (Phycoerythrin PE Cy 5), anti-Human CD 25 (AllophycocyaninAPC). Cell Acquisition and flow cytometric evaluation by FACS Calibur (BD Biosciences) was done. This was followed by cell permeabilization using FOXP3 buffer kit (BD Biosciences) for determination of intra-cellular FOXP3 levels in the same cell samples. The permeabilized samples were re-suspended using Pharmingen stain buffer and were further divided into two 300 μl suspensions and one half used for detecting the level of autofluorescence (unstained sample) and the other half of the suspension was subjected to intra-cellular staining with human FOXP3 (Alexa Fluor 488). The cell acquisition, flow cytometric analysis were repeated for the above-mentioned antibody to determine the proportion of cells expressing FOXP3. The results were analysed using the flow cytometry analysis software (FlowJo).
Statistical Analysis
Data was analysed with statistical software SPSS version 15. The mean values and frequency were calculated for the demographic variables such as age, gender, OHI (S) score, probing depth and CAL. Mean and standard deviation values for the percentage of FOXP3 expression by CD4+CD25+ cells were calculated. The difference in proportion of FOXP3 expression among the control and chronic periodontitis group were compared using non parametric Mann-Whitney Test and a p-value of <0.01 was considered statistically significant.
Results
The descriptive variables of the study population are summarized in [Table/Fig-2]. From the processed blood samples; peripheral blood mononuclear cells were gated on the basis of forward scatter (FSC), side scatter (SSC) [Table/Fig-3,4] and expression of CD 45RB was determined. This subpopulation was gated for CD 4+ and CD 25 + mononuclear cells. Cells that were CD 45RB+CD 4+CD 25+ (nTreg cells) were assessed for intra cellular Fox P3 expression following fixing and permeabilization. 43.14±11.17 % of CD 4+CD25+ cells expressed FOXP3 in group 2 as compared to 14.75±5.04 % of similar cells expressing FOXP3 in group 1, thereby demonstrating an increased proportion of nTregs in peripheral blood samples of the chronic periodontitis group [Table/Fig-5].
The demographic data of the Control (Group 1) and Chronic periodontitis (Group 2) subjects chosen for the study.
| Parameters | Group 1 | Group 2 |
|---|
| Age (years) Range Mean±SD | 26 – 31 | 26 – 56 |
| 26.46±3.1 | 36.63±9.7 |
| Gender | Male: 4 | Male: 7 |
| Female: 3 | Female: 1 |
| OHI (S) score (Mean±SD) | 0.629± 0.3 | 4.825± 0.2 |
| Probing depth (mm) (Mean±SD) | 1.286± 0.1 | 5.262± 0.4 |
| CAL (mm) (Mean±SD) | 0.00 ± .00 | 5.27 ± 0.3 |
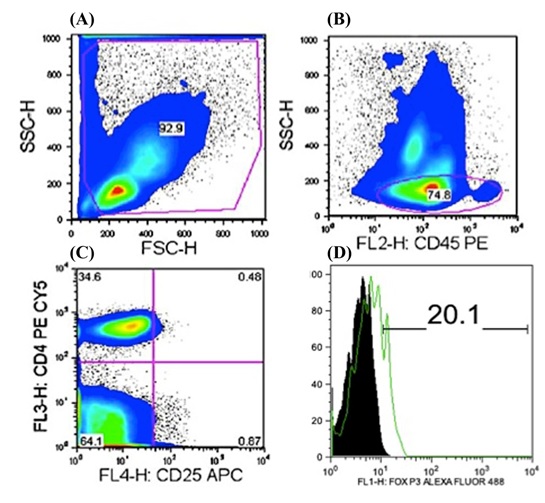
Pseudo colour plot obtained following gating (A) for lymphocyte sub population in stained control sample after fixing and permeabilization, (B) demonstrating 74.8% of CD45RB+ lymphocytes, (C) 0.48% of CD4+CD25+ lymphocytes and (D) histogram demonstrating the difference in FOXP3 expression among CD45RB+CD4+CD25+ cells in control group .
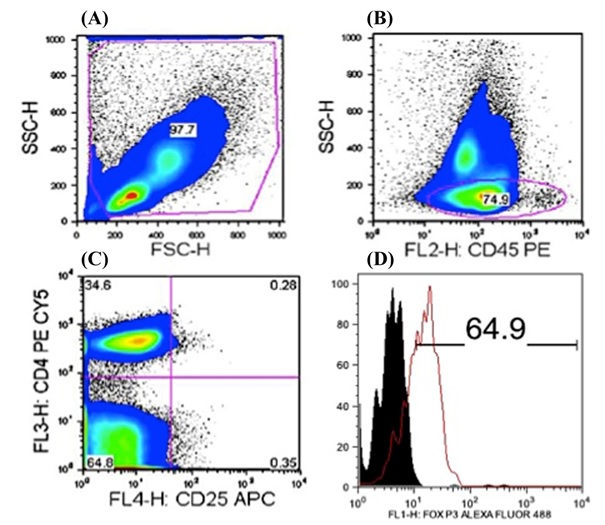
Pseudo colour plot obtained following gating (A) for lymphocyte sub population in stained periodontitis sample after fixing and permeabilization, (B) demonstrating 74.9% of CD45RB+ lymphocytes, (C) 0.28% of CD4+CD25+ lymphocytes and (D) histogram demonstrating the difference in FOXP3 expression among CD45RB+CD4+CD25+ cells in chronic periodontitis group.
Proportion of cells expressing FOXP3 in subjects with Control (Group 1) and Chronic periodontitis (Group 2) .
| CATEGORY | % of FOXP3 Expression (Mean±SD) | p-value |
|---|
| Group 1 | 14.75±5.04 | <0.001* |
| Group 2 | 43.13±11.17 |
*Mann Whitney U test, p<0.01- statistically significant
Discussion
Periodontitis is the result of an interaction between the host’s immune system and the micro-organisms present in dental plaque. Although micro-organisms play an indispensable role in the initiation of the disease, the disease progression is influenced by the host immune response amongst other factors.
T-cells, especially CD4+ T helper cells, play an indispensable role in immune homeostasis, and a disruption in the balance between micro-organisms and T-cell responses has been observed during the periodontal disease progression [15]. CD4+ T helper cells were first classified as T helper 1 (Th1) and T helper 2 (Th2) cells, based on their unique cytokine profiles and biological functions. Th1 cells secrete high levels of IFN-γ and IL-2 and are required for cell-mediated immunity. Th2 cells predominantly secrete IL-4, IL-5, and IL-13 and are required for humoral immunity. Over the past few decades, the pathogenesis of periodontal disease has been examined in the context of the Th1/Th2 paradigm; however, no consistent results have been obtained [15].
T helper 17 (Th17) cells are a recently identified subset which produces IL-17 and is involved in providing host protection against extracellular pathogens [16]. In addition, Th17 cells are considered a key T-cell subset involved in the etiology of various autoimmune and inflammatory disorders. The role of Th17 cells in the periodontal disease pathogenesis was reported by several researchers, following the observation of elevated levels of IL-17 in periodontitis lesions [17,18]. However, the origin of the Th17 cells in periodontitis lesions remains to be determined.
Tregs are a distinct subset of T cells that are required for the regulation of inflammatory responses. They play a critical role in tolerogenic responses and suppression of inflammatory responses against self-antigens. A classical characteristic of Tregs is their lack of proliferative response upon T Cell Receptor (TCR) activation and thus these cells have an anergic phenotype. The role of Tregs in the periodontitis pathogenesis is a focus of research interest. This experiment was carried out to determine the relative proportion of n Treg in peripheral blood of healthy individuals and chronic periodontitis patients. A higher proportion of CD4+ CD25+ FOXP3+ (nTreg) cells in peripheral blood were observed in the chronic periodontitis group.
Previous studies in chronic inflammatory and autoimmune conditions such as Rheumatoid arthritis [19,20] and tuberculosis [21] have reported higher levels of T regulatory cells in the systemic circulation. Conflicting reports exist in the literature regarding the role of n Treg in chronic periodontitis. Early studies by Nakajima et al., and Cardoso et al., reported an increase in the proportion of CD25+FoxP3+ cells in gingival tissue from chronic periodontitis patients [12,22]. However, a recent study has reported a higher proportion of n Tregs in healthy gingival tissue [23]. Further supportive evidence can be derived from studies on IL-35, a Treg cell specific cytokine that is required for the maximum regulatory activity of Treg cells invitro and invivo. IL-35 has been shown to be expressed by resting and activated Tregs but not by T effector (Teff) cells [24]. Nagaraj BK et al., evaluated the expression levels of IL-35 mRNA in gingival tissues of Healthy, CP, and AgP subjects [25]. The level of IL-35 mRNA was more in the chronic periodontitis subjects as compared to the aggressive periodontitis group and was least expressed among the healthy patients. The findings of our study are in agreement with those of Nakajima et al., Cardoso et al., wherein elevated levels of the T reg cells have been identified in individuals with chronic periodontitis [12,22].
To the best of our knowledge the present study is the first to determine the proportion of n Treg in peripheral blood of chronic periodontitis patients. The peripheral blood was chosen as the test sample because: a) T regulatory cells primarily remain in circulation and enter the tissues as and when the need arises; b) It has been demonstrated invitro that in humans, freshly isolated CD4+ CD25+ T-cells are susceptible to cytokine starvation-induced apoptosis [26]. Periodontitis being a chronic inflammatory disease can up regulate various cytokines in the systemic circulation. Most of the previous studies on regulatory cells in chronic periodontitis have taken into consideration CD45RO/CD45RA (naïve and memory respectively) [27–29]. To isolate active cells we used CD45RB, which is an active isoform of CD45. The exact role of T regulatory cells in chronic periodontitis pathogenesis is not yet established. In addition to the inherent suppressive activity of these cells, the conversion of these cells into a Th 17 phenotype has been demonstrated in gingival tissue from chronic periodontitis subjects [30]. However, a recent study to determine the presence of FOXP3+ Tregs and Th 17+ cells in gingival tissue specimens (from chronic periodontitis subjects) reported an increased proportion of the FOXP3+ Treg cells and lack of IL-17+/ FOXP3+ cells in the tissues examined [31].
Conclusion
The present study has definitively established that natural T regulatory cells in peripheral blood are elevated in chronic periodontitis patients and may play a potential role in pathogenesis of chronic periodontitis.
*Mann Whitney U test, p<0.01- statistically significant
[1]. Haffajee AD, Socransky SS, Microbial etiological agents of destructive periodontal diseasesPeriodontol 2000 1994 5:78-111. [Google Scholar]
[2]. Darveau RP, Hajishengallis G, Curtis MA, Porphyromonas gingivalis as a potential community activist for diseaseJ Dent Res 2012 91:816-20. [Google Scholar]
[3]. Hajishengallis G, Immunomicrobial pathogenesis of periodontitis: keystones, pathobiont and hostresponseTrends Immunol 2014 35:3-11. [Google Scholar]
[4]. Garlet GP, Destructive and protective roles of cytokines in periodontitis: host defence and tissue destruction viewpointsJ Dent Res 2010 89:1349-63. [Google Scholar]
[5]. Castro-Sanchez P, Martin-Villa JM, Gut immune system and oral toleranceBr J Nutr 2013 109(suppl 2):3-11. [Google Scholar]
[6]. Chaudhry A, Rudensky AY, Control of inflammation by integration of environmental cues by regulatory T cellsJ Clin Invest 2013 123:939-44. [Google Scholar]
[7]. Josefowicz SZ, Lu LF, Rudensky AY, Regulatory T cells: mechanisms of differentiation and functionAnnu Rev Immunol 2012 30:531-64. [Google Scholar]
[8]. Garlet GP, Sfeir CS, Little SR, Restoring host-microbe homeostasis via selective chemoattraction of TregsJ Dent Res 2014 93:834-39. [Google Scholar]
[9]. Belkaid Y, Chen W, Regulatory ripplesNat Immunol 2010 11:1077-78. [Google Scholar]
[10]. Piccirillo CA, Shevach EM, Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral toleranceSemin Immunol 2004 16:81-88. [Google Scholar]
[11]. Maggi E, Cosmi L, Liotta F, Romagnani P, Romagnani S, Annunziato F, Thymic regulatory T cellsAutoimmun Rev 2005 4:579-86. [Google Scholar]
[12]. Nakajima T, Ueki-Maruyama K, Oda T, Ohsawa Y, Ito H, Seymour GJ, Regulatory T-cells infiltrate periodontal disease tissuesJ Dent Res 2005 84:639-43. [Google Scholar]
[13]. Hori S, Nomura T, Sakaguchi S, Control of regulatory T cell development by the transcription factor Foxp3Science 2003 299:1057-61. [Google Scholar]
[14]. Armitage GC, Development of a classification system for periodontal diseases and conditionsAnn Periodontol 1999 4:1-6. [Google Scholar]
[15]. Gemmell E, Yamazaki K, Seymour GJ, The role of T cells in periodontal disease: homeostasis and autoimmunityPeriodontol 2000 2007 43:14-40. [Google Scholar]
[16]. Gaffen SL, Hajishengallis G, A new inflammatory cytokine on the block: re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17J Dent Res 2008 87:817-28. [Google Scholar]
[17]. Cardoso CR, Garlet GP, Crippa GE, Rosa AL, Junior WM, Rossi MA, Evidence of the presence of T helper type 17 cells in chronic lesions of human periodontal diseaseOral Microbiol Immunol 2009 24:1-6. [Google Scholar]
[18]. Ohyama H, Kato-Kogoe N, Kuhara A, Nishimura F, Nakasho K, Yamanegi K, The involvement of IL-23 and the Th17 pathway in periodontitisJ Dent Res 2009 88:633-38. [Google Scholar]
[19]. Kao JK, Hsue YT, Lin CY, Role of new population of peripheral CD11c+CD8+ T cells and CD4+CD25+ regulatory T cells during acute and remission stages in rheumatoid arthritis patientsJ Microbiol Immunol Infect 2007 40:419-27. [Google Scholar]
[20]. Cao D, Malmstrom V, Baecher-Allan C, Hafler D, Klareskog L, Trollmo C, Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritisEur J Immunol 2003 33:215-23. [Google Scholar]
[21]. Guyot-Revol V, Innes J A, Hackforth S, Hinks T, Lalvani A, Regulatory T cells are expanded in blood and disease sites in patients with tuberculosisAm J RespirCrit Care Med 2006 173:803-10. [Google Scholar]
[22]. Cardoso CR, Garlet GP, Moreira AP, Júnior WM, Rossi MA, Silva JS, Characterization of CD4+CD25+ natural regulatory T cells in the inflammatory infiltrate of human chronic periodontitisJ Leuk Bio 2008 84:311-18. [Google Scholar]
[23]. Ernst CW, Lee JE, Nakanishi T, Karimbux NY, Rezende TM, Stashenko P, Diminished forkhead box P3/CD25 double-positive T regulatory cells are associated with the increased nuclear factor-kappaB ligand (RANKL+) T cells in bone resorption lesion of periodontal diseaseClin Exp Immunol 2007 148:271-80. [Google Scholar]
[24]. Collison LW, Workman CJ, Kuo TT, The inhibitory cytokine IL-35 contributes to regulatory T-cell functionNature 2007 450:566-69. [Google Scholar]
[25]. Nagaraj BK, Akshay M, Shivaprasad BM, Arati CK, Expression Profile of IL-35 mRNA in Gingiva of Chronic Periodontitis and Aggressive Periodontitis Patients: A Semiquantitative RT-PCR StudyDisease Markers 2013 35:819-23. [Google Scholar]
[26]. Akbar AN, Taams LS, Salmon M, Vukmanovic-Stejic M, The peripheral generation of CD4+ CD25+ regulatory T cellsImmunology 2003 109:319-25. [Google Scholar]
[27]. Fehervari Z, Sakaguchi S, CD4+ Tregs and immune controlJ Clin Invest 2004 114:1209-17. [Google Scholar]
[28]. Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation toleranceImmunol Rev 2001 182:18-32. [Google Scholar]
[29]. Shevach EM, CD4+ CD25+ suppressor T cells: more questions than answersNat Rev Immunol 2002 2:389-400. [Google Scholar]
[30]. Okui T, Aoki Y, Ito H, Honda T, Yamazaki K, The presence of IL-17+/FOXP3+ double-positive cells in periodontitisJ Dent Res 2012 91:574-79. [Google Scholar]
[31]. Parachuru VP, Coates DE, Milne TJ, Hussaini HM, Rich AM, Seymour GJ, Forkhead box P3-positive regulatory T-cells and interleukin 17-positive T-helper 17 cells in chronic inflammatory periodontal diseaseJ Periodontal Res 2014 49:817-26. [Google Scholar]