Introduction
Dyslipidaemia of Nephrotic Syndrome (NS) is known to be linked to oxidative reactions and atherosclerosis. Paraoxonase (PON1) has been implicated in the prevention of Low Density Lipoprotein (LDL) lipid peroxidation and also degrades biologically active oxidised lipids in lipoprotein.
Aim
The present study was taken up to assess PON1 levels in paediatric nephrotic syndrome and also to see if any correlation exists between lipid parameters and PON1.
Materials and Methods
This study consists of Group 1 with 40 cases of NS in the age group of 2-14 years and Group 2 with 40 age and sex matched healthy controls. Lipid profile and paraoxonase activity was measured in serum samples of both the groups.
Results
Statistical analysis by student’s t-test showed that the mean levels of Total Cholesterol, Trigylycerides, LDL, and VLDL were significantly increased in Group 1 when compared to Group 2 (p <0.001). The mean levels of HDL were similar in both groups. The levels of PON1 were significantly lowered in Group 1 when compared to Group 2. Correlation studies showed no significant correlation between lipid profile and PON1.
Conclusion
Cases have atherosclerotic dyslipidaemia and significantly decreased PON1 activity. Decreased PON1 may lead to increased oxidation of LDL accelerating the process of atherosclerosis.
Introduction
Hyperlipidaemia has been recognized as a common finding in nephrotic patients since 1917. The lipid profile seen in Nephrotic Syndrome (NS) is atherogenic and it predisposes to premature Coronary Artery Disease (CAD) [1,2]. Hyperlipidaemia is not always connected with nephrotic disease activity and may sometimes persist for long time during remission, especially in frequently relapsing NS which worsens further prognosis [3].
Human Serum Paraoxonase (PON1) is tightly associated with apolipoprotein A1 in High Density Lipoprotein (HDL) and has highest activity in the liver and blood. PON1 has been implicated in the prevention of Low Density Lipoprotein (LDL) lipid peroxidation and also degrades biologically active oxidized lipids in lipoprotein [4,5]. Oxidized LDL formation in the sub endothelial space of arterial wall is a key initial step in atherosclerosis. Moreover, there is an inverse relationship between the level of oxidized lipid products and PON1 activity. There is reduced activity of PON1 in subjects who are at high risk of developing atherosclerosis [6,7]. NS in adulthood is associated with an increased risk of atherosclerosis and coronary heart disease [8]. HDL from PON1 knockout mice cannot prevent oxidation of LDL in a co-culture model (in mice liver cells and HepG2 Human Hepatoma cell lines) simulating the artery wall and their macrophages contain more oxidized lipid. The same is true in human population studies [9]. One prospective study has shown that reduced serum PON1 activity was an independent predictor of coronary disease [10]. Dyslipidaemia of NS is known to be linked to oxidative reactions. The oxidative reactions cause endothelial dysfunction, increased lipid oxidation. There is impaired activity of some antioxidant enzymes in the acute phase of idiopathic NS i.e., PON1, that may increase the risk of development of atherosclerosis [1].
It is important to know the antioxidant defense status i.e., PON1 activity in NS patients. Very few studies have investigated the levels PON1 activity and its relation to dyslipidaemia in paediatric NS in Indian population. There is dramatic improvement in the life expectancy of children afflicted with NS over the last 15 years, so, the occurrence of dyslipidaemia with its associated morbidity is of particular concern.
Aim
Hence, the present study was taken up with the aim to study the derangement of serum lipids and PON1 activity in nephrotic syndrome and also to know if any correlation exists between the lipid parameters and PON1 activity.
Materials and Methods
It was a hospital based cross-sectional comparative study. The study was conducted in Department of Biochemistry, Jawaharlal Nehru Medical College, Belgaum, over a period of twelve months from February 2010 to February 2011.
Study Participants and Groups
The study participants included two groups, Group 1 consisted of 40 cases of NS patients in the age group of 2 to 14 years of both sex and Group 2 consisted of 40 age and sex matched healthy controls.
Inclusion Criteria
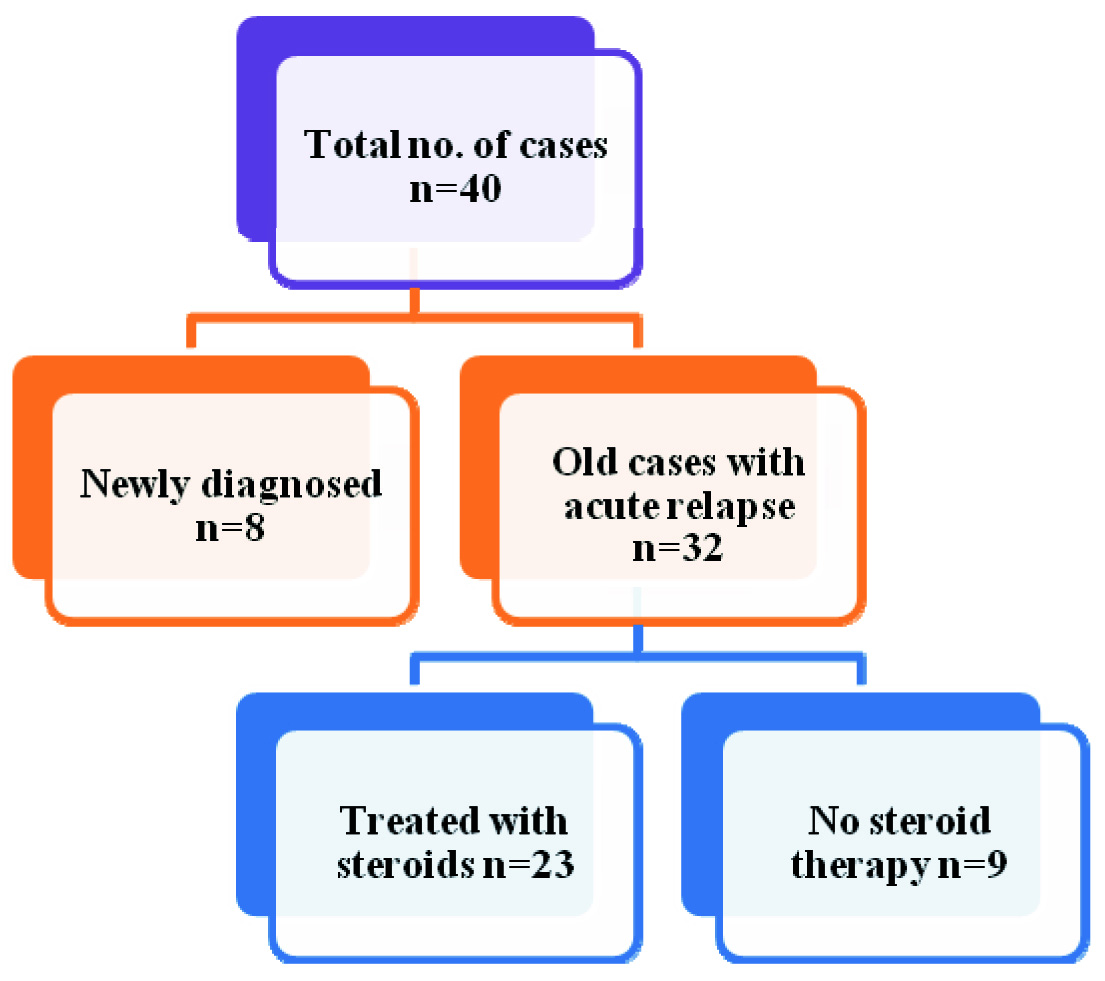
Group 1 comprised of clinically, diagnostically confirmed cases of NS in active phase (both newly diagnosed and old cases with acute relapse), age 2 to 14 years of either sex admitted or attending the paediatric unit of KLES Dr Prabhakar Kore Hospital and Medical Research Centre, Belgaum. Group 2 consisted of age and sex matched healthy children who were selected from among those who came for routine immunization and follow up in the paediatric Out Patient Department (OPD).
Exclusion Criteria
Familial hyperlipidaemia, patients with other renal pathology, patients with liver disease, and malnourished children were excluded from the study.
Ethical Approval
Ethical clearance has been obtained from the Institutional Ethical Clearance Committee, J. N. Medical College, Belgaum. The study was conducted in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. An informed consent was obtained from parents of patients and control group before collecting the blood sample.
Patient details, clinical details and lab results were noted in the proforma. We noted down the duration of illness, the number of relapses the child had in the past, treatment history - whether the child was given steroid therapy, compliance for the same was noted for each patient to look for its correlation with PON1 [Table/Fig-1].
Categorization of Study participants in Group 1
Sample collection
5ml of venous blood was collected from the patients and controls under aseptic precautionary measures using disposable syringe in plain tubes. The blood was allowed to clot for 30 minutes. Serum was then separated by centrifugation for 10 minutes at 2000 rpm within one hour of collection and analysed within 24 hours or kept at -200C if analysis was delayed.
Method of Assay
The following parameters were analysed from the serum sample: Total cholesterol (TC) by CHOD/PAP Enzymatic method [11]; HDL-C by Polyethylene glycol precipitation method [12]; Triglycerides (TG) by GPO-PAP enzymatic method [13]. LDL, VLDL – calculated by Friedewald’s formula [14].
The analysis was done spectrophotometrically using coral reagent kits from CREST BIOSYSTEMS, INDIA.
PON1 activity was tested by Spectrophotometric method using P-nitrophenyl acetate (from ACROS ORGANICS) as a substrate. The increase in the absorbance at 402 nm due to formation of p-nitrophenol was measured. 50μL serum was added to 2ml of Tris-HCl buffer (25mM, pH 7.4, 25°C) containing 1.0mM CaCI2, and 5.5 mM/L p-nitrophenyl acetate. For non-enzymatic hydrolysis of p-nitrophenyl acetate, 2 ml of buffer was taken in spectrophotometric cuvette and 50 μl of substrate was added to it. The rate of change in absorbance (A) was monitored at 30, 60, 90,120 and 150 seconds at 402 nm. The ΔA (rate of change in absorbance)/min was calculated. The difference in absorbance was calculated by deducting the ΔA obtained by non enzymatic hydrolysis of substrate from ΔA obtained in the presence of serum. The calculation was done omitting the first 30 seconds in order to allow the reaction to reach a steady state. The molar absorptivity of 14000 M-1 cm-1 at pH 7.4 for the substrate p-nitrophenyl acetate was used to calculate the PON1 activity in U/L [15].
Statistical Analysis
The data was analysed by SPSS software. Results were expressed as Mean ± SD. Student’s t-test and chi square test were used for group wise comparisons. Relationship between variables was measured by Karl Pearson’s correlation coefficient. A statistical significance was set at 5% level of significance (p<0.05).
Results
Demographic parameters of both Group1 and Group 2 are shown in [Table/Fig-2]. There was no statistical difference in the sex distribution (p-value 0.178) and mean age between the two groups both the groups were comparable.
Comparison of demographic parameters in Group 1 and Group 2.
| Parameters | Group 1 | Group 2 |
|---|
| Age (in years ± SD) | 6.2 ± 3.2 | 7.1 ± 2.4# |
| Males (n) | 25 | 19# |
| Females(n) | 15 | 21# |
#p>0.05 (not significant)
The biochemical parameters i.e., lipid profile and PON1 of both the groups are shown in [Table/Fig-3]. Statistical analysis by student’s t-test showed that the mean levels of TC, TG, LDL, and VLDL were significantly increased in Group1 when compared to Group 2 (p <0.001). The mean levels of HDL were similar in both groups as shown in [Table/Fig-3]. The levels of PON1 are significantly lowered in Group 1 when compared to Group 2 (p<0.001).
Comparison of lipid profile and PON1 in Group1 and Group 2 (values expressed as mean ± SD).
| Parameters | Group 1 (n=40) | Group 2 (n=40) | p |
|---|
| Total cholesterol (mg/dL) | 364 ± 94.6 | 168.0 ± 24.5** | <0.001 |
| Triglycerides (mg/dL) | 285.8 ± 88.2 | 129.4 ± 29.6** | <0.001 |
| HDL (mg/dL) | 50.7 ± 15.5 | 49.3 ± 6.0# | >0.05 |
| LDL (mg/dL) | 256.7 ± 83.3 | 92.4 ± 24.1** | <0.001 |
| VLDL (mg/dL) | 57.8 ± 18.4 | 26.0 ± 5.8** | <0.001 |
| PON1 (IU/L) | 218.7 ± 35.3 | 302.3 ± 43.4** | <0.001 |
#p>0.05 (not significant), *p<0.05 (significant), **p<0.001 (highly significant)
We also looked for any effect of steroid therapy in the past on PON1 activity in the acute phase of NS. Comparative analysis of PON1 in old cases of NS with acute relapse among the subjects from group1, who were treated with steroids (n=23) and cases who did not receive steroid therapy in the past (n=09) showed that the levels of PON1 were significantly higher in cases who have not received steroid therapy in comparison to patients who received steroid therapy with a p-value of 0.033 as shown in [Table/Fig-4].
Comparison of PON1 with respect to steroid therapy received in the past among old cases in Group1.
| Steroid therapy in the past | Received (n=23) | Not received (n=09) | p |
|---|
| PON1 (IU/L)± SD | 212.0 ± 32.2 | 238.0 ± 20.5* | <0.05 |
*p<0.05 (significant)
We found no significant correlation between lipid profile parameters- TC, TG, HDL, LDL, VLDL and PON1 (p>0.05) within the groups. But the relation was different in both the groups. Group 1 showed a negative correlation between non HDL lipids and PON1, whereas Group 2 showed a positive relation. HDL correlated positively with PON1 in Group 1, whereas the relation was negative in case of Group 2 as shown in [Table/Fig-5]. Additionally, when we looked for correlation between duration of illness and PON1 in Group 1, we found no significant correlation (p>0.05). [Table/Fig-6] shows the correlation based on the duration of illness.
Correlation between lipid profile and PON1 in Group 1 and Group 2.
| Parameters | PON1 in Group 1 (n=40) r | p | PON1 in Group 2 (n=40) r | p |
|---|
| Total cholesterol (mg/dL) | -0.22 | 0.16# | 0.08 | 0.62# |
| Triglycerides (mg/dL) | -0.25 | 0.12# | 0.25 | 0.13# |
| HDL (mg/dL) | 0.10 | 0.53# | -0.09 | 0.60# |
| LDL (mg/dL) | -0.21 | 0.18# | 0.06 | 0.72# |
| VLDL (mg/dL) | -0.26 | 0.10# | 0.26 | 0.11# |
#p>0.05 (not significant)
Correlation between duration of illness (in years) and POM and in Group1.
| Variables | r | p |
|---|
| Duration of illness vs POM | -0.1802 | 0.2659# |
#p>0.05 (not significant)
Discussion
In agreement with previous studies [1,16–19] our subjects in Group 1 had significantly increased levels of TC, TG, LDL and VLDL in comparison to Group 2 (p <0.001). In contrast to the study done by El-Melegy et al., who found lower levels of HDL in cases, we found no significant differences in the mean levels of HDL between Group 1 and Group 2 [1]. This is in accordance to study done by Muls et al., who found normal levels of HDL in NS [20]. They also reported abnormal distribution of HDL subtypes. Also, Hu P et al., observed higher levels of HDL in nephrotic cases compared to controls [19]. There is increased levels of HDL3 and decreased HDL2 [20–22] which measured together give normal values for total HDL, but result in defective reverse cholesterol transport pathway.
Hyperlipidaemia and dyslipidaemia in NS occurs due to abnormalities in lipoprotein metabolism i.e. increased synthesis of VLDL, LDL, decreased catabolism of VLDL and LDL and altered reverse cholesterol transport pathways. The exact cause for these disturbances is not yet clear [8].
Further, we also found in this study, the levels of enzyme PON1 that hydrolyses oxidized lipids, is decreased significantly in Group 1 when compared to Group 2 as shown in [Table/Fig-3]. This is in accordance to studies done by researchers Kniażewska MH and Ece et al., [16,23]. The phenotypic changes induced by hyperlipidaemia in microcirculation leads to platelet activation, lipid peroxidation and generation of radicals; the mediator being oxidative and nitrosative stresses which along with impaired activity of some antioxidant enzymes-PON1 in the acute phase of NS increase the risk of early atherosclerosis development [1,16,23–26]. Pathology studies have established that atherosclerotic disease begins in childhood and will progress faster if risk factors are present [27]. With hyperlipidaemia in NS, there is increased accumulation of lipoproteins in intima of blood vessels which due to changes induced by free radicals get oxidized to ox LDL and ingested by macrophages via scavenger receptors forming foam cells. Once ox LDL saturates macrophages and smooth muscle cells start accumulating lipids, the process of atherosclerosis becomes progressive and cannot be reversed back [27].
The formation of biologically active oxidized lipids can be prevented by the antioxidants present in LDL and the microenvironment where LDL is trapped. The decrease in PON1 levels leads to elevated levels of ox LDL, as the antioxidant activity of the HDL is reduced. This results in increased lipid peroxidation of lipoproteins and elevated levels of lipid hydroperoxides with decreased hydrolysis of ox LDL predisposing to the risk of atherosclerosis [25,28–30]. This relationship between PON1 activity and lipid peroxidation of lipoproteins is also confirmed by another study which established a negative correlation between HDL-PON1 activity and the levels of lipid hydroperoxides associated with HDL and LDL [30].
Additionally, in our study we found the levels of PON1 are significantly higher in active cases who have not received steroid therapy in the past in comparison to patients who received steroid therapy (among the old cases of Group 1) as seen in [Table/Fig-4]. This suggests that steroids will neither suppress the oxidative stress nor correct the lipid abnormalities in NS [23] and some other modality of treatment is required to prevent formation of oxidized lipids. The results of study done by researchers suggest that nephrotic children may have prolonged periods of hyperlipidaemia even after clinical remission [31]. The abnormal lipid profile is more likely seen in children with frequent relapses even during their remission [32]. Lipid abnormalities seen in NS constitutes atherogenic dyslipidaemia and can predispose to subclinical atherosclerosis in adulthood. This necessitates the coronary artery disease risk assessment and intervention in paediatric population [33].
We found no significant correlation between TC, TG, LDL, VLDL and PON1 in both Group 1 and Group 2. This is in accordance to a study done in healthy children in Slovakia who found no correlation between PON1 and lipid parameters [7]. We found no significant correlation between HDL and PON1 in Group 1 and Group 2. Some studies have shown a positive correlation between HDL and PON1 [4,23,34,35]. In a study in adult population there was a positive correlation between PON1 and LDL, VLDL in cases with vascular diseases and in controls PON1 correlated with HDL [36]. The relationship of PON1 and lipid profile is different in Group 1 versus Group 2 in our study though not statistically significant [Table/Fig-5], suggests other factors influenced PON1 activity more in cases than in controls. One of these factors may be the co-regulation of the PON1 activity with lipoprotein levels. The joint regulation of PON1 with lipoproteins differs in these two groups, suggesting PON1 activity predicts risk of atherosclerosis.
Limitations
HDL subtypes were not estimated separately. Ox LDL which would be a better marker to estimate the risk of atherosclerosis and correlate with PON1 activity was not measured due to cost reasons.
Conclusion
In total the cases of NS had significant increased levels of all lipids except for HDL cholesterol. Also PON1 activity is significantly decreased in these cases indicating decreased antioxidant capacity to prevent lipid oxidation. Steroid therapy in the past for relapsers did not improve lipid profile or PON1 levels in acute relapse. Hyperlipidaemia with decreased antioxidant capacity due to reduced PON1 collectively predisposes the NS patients to the risk of atherosclerosis. PON1 could be used as a marker along with Lipid profile to assess risk for atherosclerosis in NS.
Thus, it can be stated that Nephrotic syndrome is associated with dyslipidaemia and decreased levels of PON1, which may accelerate the development of atherosclerosis and thus requires monitoring of these parameters and finding new approaches towards treating them.
#p>0.05 (not significant)
#p>0.05 (not significant), *p<0.05 (significant), **p<0.001 (highly significant)
*p<0.05 (significant)
#p>0.05 (not significant)
#p>0.05 (not significant)
[1]. El-Melegy NT, Mohamed NA, Sayed MM, Oxidative modification of low-density lipoprotein in relation to dyslipidaemia and oxidant status in children with steroid sensitive nephrotic syndromePaediatr Res 2008 63(4):404-09. [Google Scholar]
[2]. Grundy SM, Atherogenic dyslipidaemia: lipoprotein abnormalities and implications for therapyAm J Cardiol 1995 75(6):45B-52B. [Google Scholar]
[3]. Ksiazek J, Ciechanowicz A, Wierzbicka A, Syczewska M, Grenda R, Is dyslipidaemia sustained during remission of nephrotic syndrome genetically determined? Evaluation of genetic polymorphisms of proteins involved in lipoprotein metabolism in children and adolescents with nephrotic syndromePol Arch Med Wewn 2009 119(1-2):11-16. [Google Scholar]
[4]. Abbott CA, Mackness MI, Kumar S, Boulton AJ, Durrington PN, Serum paraoxonase activity, concentration, and phenotype distribution in diabetes mellitus and its relationship to serum lipids and lipoproteinsArterioscler Thromb Vasc Biol 1995 15(11):1812-18. [Google Scholar]
[5]. Gur M, Yildiz A, Demirbag R, Yilmaz R, Aslan M, Ozdogru I, Paraoxonase and arylesterase activities in patients with cardiac syndrome X, and their relationship with oxidative stress markersCoron Artery Dis 2007 18(2):89-95. [Google Scholar]
[6]. James RW, A long and winding road: defining the biological role and clinical importance of ParaoxonasesClin Chem Lab Med 2006 44(9):1052-59. [Google Scholar]
[7]. Sumegova K, Nagyova Z, Waczulikova I, Zitnanova I, Durackova Z, Activity of Paraoxonase1 And Lipid Profile In Healthy ChildrenPhysiol. Res 2007 56:351-57. [Google Scholar]
[8]. Eddy AA, Symons JM, Nephrotic syndrome in childhoodLancet 2003 62(9384):629-39. [Google Scholar]
[9]. Getz GS, Catherine A, Paraoxonase, a cardioprotective enzyme: continuing issuesReardon Curr Opin Lipidol 2004 15:261-67. [Google Scholar]
[10]. Mackness B, Durrington P, McElduff P, Yarnell J, Azam N, Watt M, Low paraoxonase Activity Predicts Coronary Events in the Caerphilly Prospective StudyCirculation 2003 107:2775-79. [Google Scholar]
[11]. Allain CC, Poon LS, Chan CS, Richmond W, Fu PC, Enzymatic determination of total serum cholesterolClin Cem 1974 20(4):470-75. [Google Scholar]
[12]. Warnick GR, Nguyen T, Albers AA, Comparison of improved precipitation methods for quantification of high-density lipoprotein cholesterolClin Chem 1985 31(2):217-22. [Google Scholar]
[13]. Bucolo G, David H, Quantitative determination of serum Triglycerides by the use of enzymesClin Chem 1973 19(5):476-82. [Google Scholar]
[14]. Friedewald WT, Levy RI, Fredrickson DS, Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifugeClin Chem 1972 18(6):499-502. [Google Scholar]
[15]. Dantoine TF, Debord J, Charmes JP, Merle L, Marquet P, Lachatre G, Decrease of serum paraoxonase activity in chronic renal failureJ Am Soc Nephrol 1998 9(11):2082-88. [Google Scholar]
[16]. Kniazewska MH, Obuchowicz AK, Wielkoszynski T, Zmudzinska-Kitczak J, Urban K, Hyla-Klekot L, Evaluation of certain constituents of antioxidant defense in youth treated in the past for steroid-sensitive idiopathic nephrotic syndromePaediatr Nephrol 2009 24(11):2187-92. [Google Scholar]
[17]. Querfeld U, Gnasso A, Haberbosch W, Augustin J, Schärer K, Lipoprotein profiles at different stages of the nephrotic syndromeEur J Paediatr 1988 147(3):233-38. [Google Scholar]
[18]. Poloczek BS, Tomasik A, Tarnawski R, Hyla-Klekot L, Dyduch A, Wojciechowska C, Nephrotic origin hyperlipidaemia, relative reduction of vitamin E level and subsequent oxidative stress may promote atherosclerosisNephron 2001 89(1):68-72. [Google Scholar]
[19]. Hu P, Lu L, Hu B, Du PF, Characteristics of lipid metabolism under different urinary protein excretion in children with primary nephrotic syndromeSc and J Clin Lab Invest 2009 69(6):680-86. [Google Scholar]
[20]. Muls E, Rosseneu M, Daneels R, Schurgers M, Boelaert J, Lipoprotein distribution and composition in the human nephrotic syndromeAtherosclerosis 1985 54(2):225-37. [Google Scholar]
[21]. Attman PO, Alaupovic P, Pathogenesis of hyperlipidaemia in nephrotic syndromeAm J Nephrol 1990 10(1):69-75. [Google Scholar]
[22]. Wheeler DC, Bernard DB, Lipid abnormalities in the nephrotic syndrome: causes, consequences, and treatmentAm J Kidney Dis 1994 23(3):331-46. [Google Scholar]
[23]. Ece A, Atamer Y, Gürkan F, Davutoğlu M, Koçyiğit Y, Tutanç M, Paraoxonase, total antioxidant response, and peroxide levels in children with steroid-sensitive nephrotic syndromePaediatr Nephrol 2005 20:1279-84. [Google Scholar]
[24]. Tkaczyk M, Czupryniak A, Owczarek D, Lukamowicz J, Nowicki M, Markers of Endothelial Dysfunction in Children with Idiopathic Nephrotic SyndromeAm J Nephrol 2008 28:197-202. [Google Scholar]
[25]. Navab M, Berliner JA, Watson AD, Hama SY, Territo MC, Lusis AJ, The Yin and Yang of oxidation in the development of the fatty streak. A review based on the 1994 George Lyman Duff Memorial LectureArterioscler Thromb Vasc Biol 1996 16(7):831-42. [Google Scholar]
[26]. Bakr A, Hassan SA, Shoker M, Zaki M, Hassan R, Oxidant stress in primary nephrotic syndrome:does it modulate the response to corticosteroids?Paediatr Nephrol 2009 24:2375-80. [Google Scholar]
[27]. Schoen FJ, Blood Vessels. Kumar V, Abbas AK, Fausto N. editorsRobbins and Cotron Pathologic basis of disease 2004 7th editionPhiladelphiaSaunders Elsevier [Google Scholar]
[28]. Biyikli NK, Alpay H, Yildiz N, Agachan B, Ergen A, Zeybek U, Paraoxonase 1 192 and 55 polymorphisms in nephrotic childrenPaediatr Nephrol 2006 21:649-54. [Google Scholar]
[29]. Ferretti G, Bacchetti T, Moroni C, Savino S, Liuzzi A, Balzola F, Paraoxonase Activity in High-Density Lipoproteins: A Comparison between Healthy and Obese FemalesJ Clin Endocrinol Metab 2005 90:1728-33. [Google Scholar]
[30]. Hashemi M, Sadeghi-Bojd S, Raeisi M, Moazeni-Roodi A, Evaluation of Paraoxonase Activity in children with Nephrotic SyndromeNephrourol Mon 2013 5(5):978-82. [Google Scholar]
[31]. Zilleruelo G, Hsia SL, Freundlich M, Gorman HM, Strauss J, Persistence of serum lipid abnormalities in children with idiopathic nephrotic syndromeJ Paediatr 1984 104(1):61-64. [Google Scholar]
[32]. Merouani A, Levy E, Mongeau JG, Robitaille P, Lambert M, Delvin EE, Hyperlipidemic profiles during remission in childhood idiopathic nephrotic syndromeClin Biochem 2003 36(7):571-74. [Google Scholar]
[33]. Frontini MG, Srinivasan SR, Xu J, Tang R, Bond MG, Berenson GS, Usefulness of Childhood Non–High Density Lipoprotein Cholesterol Levels Versus Other Lipoprotein Measures in Predicting Adult Subclinical Atherosclerosis: The Bogalusa Heart StudyPaediatrics 2008 121:924-29. [Google Scholar]
[34]. Sarkar PD, Shivaprakash TM, Madhusudhan B, Association between Paraoxonase activity and lipid levels in patients with premature coronary artery diseaseClinica Chimica Acta 2006 373:77-81. [Google Scholar]
[35]. Mackness MI, Abbot CA, Arrol S, Durrington PN, The role of high density lipoprotein and lipid soluble antioxidant vitamins in inhibiting low-density lipoprotein oxidationBiochem J 1993 294:829-35. [Google Scholar]
[36]. Rozek LS, Hatsukami TS, Richter RJ, Ranchalis J, Nakayama K, McKinstry LA, The correlation of Paraoxonase (P0N1) activity with lipid and lipoprotein levels differs with vascular disease statusJ Lipid Res 2005 46:1888-95. [Google Scholar]