Pregnancy is known to create profound changes in the body. It not only increases demand for metabolic fuels for the foetal growth and development of its associated structures, but also causes hormonal changes in the body which may lead to changes in lipid profile during different trimesters of the pregnancy [1].
It has been noted that in first trimester, the maternal metabolic environment gets modified due to rise in serum levels of oestrogen, and progesterone followed by pancreatic beta-cell hyperplasia leading to increase in insulin secretion [2].
Hyperinsulinaemia leads to a decline in serum glucose level by increasing the peripheral utilisation of glucose followed by its storage in tissues in form of glycogen. It also increases the storage of fat while a decline in lipolysis has been noted as well [3].
During middle and last trimester maternal fuel adjustments occurs which leads to the sparing of glucose (for the foetus) and an increased concentration of fatty acids in plasma leading to GDM and HTN respectively. Freinkel had described these changes as “accelerated starvation”, and “facilitated anabolism” [4].
GDM and HTN can contribute to maternal and foetal risk of developing peri and postpartum complications [5,6]. The third component of the metabolic syndrome associated with insulin resistance, i.e., dyslipidaemia, is a well-known cardiovascular risk factor.
However, lipid profile during second and third trimester of pregnancy has not been studied extensively in large population-based cohorts in developing countries like India. Serum levels of total cholesterol, triglyceride, high density lipoprotein and low-density lipoprotein during second and third trimesters of pregnancy and their changes with gestational age are not well described.
Hence, the present study was undertaken to find out whether there is any significant variation in the lipid profile during the second and third trimesters of a normal pregnancy and to establish a relation of pregnancy with its effects on lipid profile.
Materials and Methods
This was a prospective study conducted at Mahatma Gandhi Mission Hospital, Navi Mumbai, India. A total of 200 pregnant local women were enrolling who visited the hospital from October 2012 to 2014. Out of the 200 enrolled subjects, 10 of them developed gestational hypertension in late third trimester which was detected after 32nd week during followup. But these patients were also included. All the women signed informed consent form before being enrolled and were followed upto delivery at MGM Hospital. There were no drop outs neither any patient was lost to follow up.
A detailed history about present pregnancy, history of diabetes, renal disorders, thyroid disorders, family history regarding preeclampsia was taken before enrolling patients for the study. Subject’s body mass index was calculated on enrolment and those who were obese were excluded from the study.
Under all aseptic precautions about 10 ml of venous blood sample was collected from all enrolled cases for measurement of lipid profile in second trimester in the 16th week and it was collected in third trimester in the 32nd week for analysis.
The samples were processed at the Central Laboratory of MGM Hospital and it was sponsored by the Department of Medicine of MGM Medical College and Hospital. Serum Cholesterol, HDL-Cholesterol were analysed on AU480 biochemistry autoanalyser by CHO-POD method. Triglycerides were analysed on AU480 biochemistry autoanalyser by GPO-POD Method. The sample values were compared with the values of the third report of the Expert Panel on Detection, Evaluation, and Treatment of high blood cholesterol in adults (Adult Treatment Panel III, or ATP III) of National Cholesterol Education Program’s (NCEP’s) updated recommendations for cholesterol testing and management.
Inclusion Criteria: All pregnant women with a singleton pregnancy who came to our hospital with gestational age of 13-28 weeks as determined by last menstrual period or ultra-sound scan, irrespective of parity and gravida were included.
Exclusion Criteria: We excluded pregnant women in whom hypertension was detected before 14 weeks. Pregnant women with diseases or complications like chronic hypertension, Diabetes, Renal Disorders and Thyroid Disorders, Obstetric and Foetal Complications (Hydrops foetalis, congenital foetal anomalies) were also excluded.
Ethics: The procedures followed were in accordance with the ethical standards of this Institution’s committee on human experimentation (institutional) and with the Helsinki Declaration of 1975 that was revised in 2000. The investigations were funded by the Department of Medicine, MGM Medical College as a part of research studies.
Statistical Analysis
The data was entered into MS-Excel sheet and statistical analyses were performed using Statistical Package for Social Sciences (SPSS) version 17. All reported p-values are two-tailed, and confidence intervals were calculated at the 95% level. The data was presented using frequencies, percentages, descriptive statistics followed by charts and graphs.
Results
Serum Cholesterol
The mean age of patients was 24.87 years with a SD of 2.7 years. The minimum age was 18 years and the maximum age was 30 years.
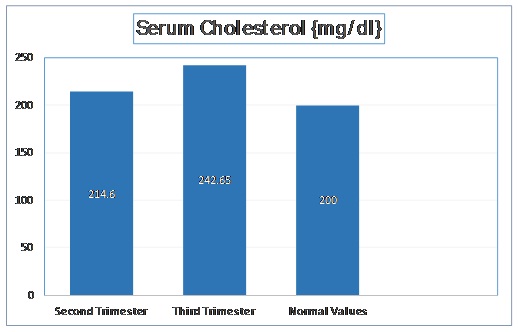
The mean cholesterol level in second trimester was 214.6 mg/dl with a standard deviation of 18.16 mg/dl. In third trimester, the mean cholesterol level was increased to 242.65 mg/dl with a standard deviation of 20.44 mg/dl. The t-stat value was found to be -14.51 (p < 0.001). These values are depicted in [Table/Fig-1].
Normal Values of Serum Cholesterol as per Adult Treatment Panel III of National Cholesterol Education Program.
Serum Triglycerides Levels
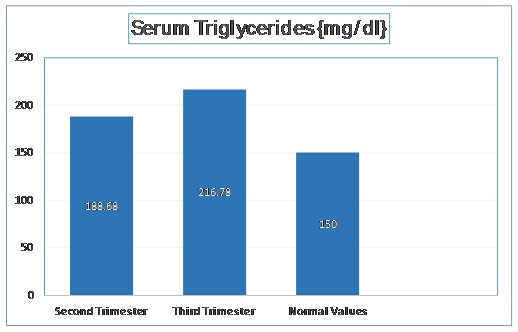
The mean triglyceride level in second trimester was 188.68 mg/dl with a standard deviation of 20.88 mg/dl. In third trimester, the mean triglyceride level was increased to 216.78 mg/dl with a standard deviation of 20.09 mg/dl. The t-stat value was found to be -13.715 (p < 0.001). These values have been depicted in [Table/Fig-2].
Normal Values of Serum Triglycerides as per Adult Treatment Panel III of National Cholesterol Education Program
HDL-Cholesterol Levels
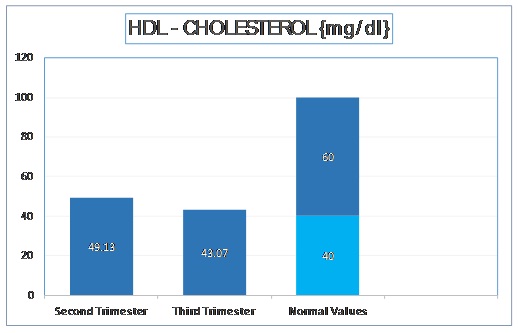
The mean HDL – Cholesterol level in second trimester was 49.13 mg/dl with a standard deviation of 6.15 mg/dl.
In third trimester, the mean HDL Cholesterol was decreased to 43.07 mg/dl with a standard deviation of 4.34 mg/dl. The t-stat value was found to be 11.368 (p < 0.001). These values are depicted in [Table/Fig-3].
Normal Values of Serum HDL - Cholesterol as per Adult Treatment Panel III of National Cholesterol Education Program
LDL-Cholesterol Levels
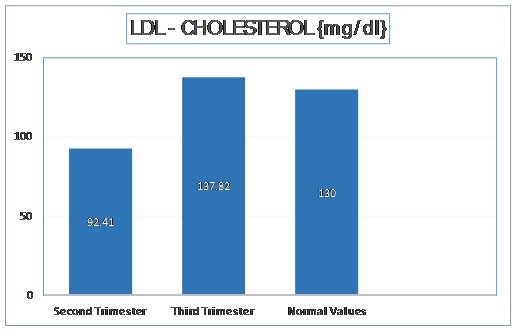
The mean LDL – Cholesterol level in second trimester was 92.41 mg/dl with a standard deviation of 18.94 mg/dl. In third trimester, the mean LDL Cholesterol was increased to 137.82 mg/dl with a standard deviation of 13.45 mg/dl. The t-stat value was found to be -27.643 (p < 0.001). These values have been depicted in [Table/Fig-4].
Normal Values of Serum LDL - Cholesterol as per Adult Treatment Panel III of National Cholesterol Education Program
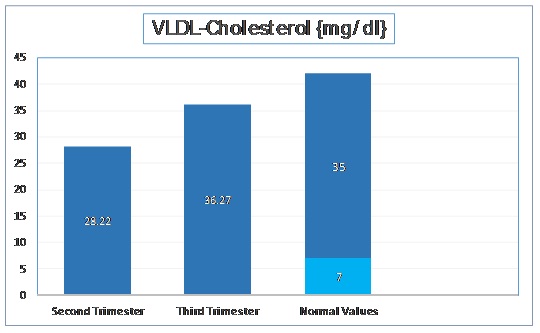
VLDL-Cholesterol Levels
The mean VLDL – Cholesterol level in second trimester was 28.22 mg/dl with a standard deviation of 7.66 mg/dl. In third trimester, the mean VLDL Cholesterol was increased to 36.27 mg/dl with a standard deviation of 6.72 mg/dl. The t-stat value was found to be -11.181 (p < 0.001). These values have been depicted in [Table/Fig-5].
Normal Values of Serum VLDL - Cholesterol as per Adult Treatment Panel III of National Cholesterol Education Program
Discussion
In pregnancy we see profound anatomic and physiologic changes in almost every organ system. These changes start occurring just after conception have taken place and keeps on evolving throughout the pregnancy including the delivery period. These changes occur in order to facilitate the needs of mother and foetus.
Maternal physiology is highly influenced by the placental hormones especially in last trimester of the pregnancy. The variation in hormonal levels generally affects the glucose and lipid metabolism and such variations take place in order to make sure that the foetus receives an ample supply of nutrients for its development [7,8].
Moreover, deposition of proteins, fats and water in the intracellular compartment of the body leads to physiological maternal weight gain. Due to increase in the metabolic requirements during pregnancy, the maternal body responds by switching over to fat utilisation from that of carbohydrate. Increase in insulin resistance and plasma lipolytic hormonal concentration also facilitates it [7,8]. These changes leads to large variations in insulin and glucose levels in the mother as she oscillates from fed to fasted state. During the fasted state, the glucose is preserved for foetus while alternative sources of fuels are made available to the mother for her metabolic use. After an overnight fast the maternal fasting capillary whole blood glucose concentration falls, while plasma ketone and free fatty acid concentrations rise [5,8].
Serum total cholesterol and triglyceride concentrations increase markedly during pregnancy, but reported ranges vary among studies [9–12].
The large rise in triglycerides is due to two factors, increased hepatic lipase activity, leading to enhanced hepatic triglyceride synthesis and reduced lipoprotein lipase activity, resulting in decreased catabolism of adipose tissue [13]. The white adipose tissue is a very active endocrine organ, which also releases a number of endocrine and paracrine factors termed adipokines [11]. Apolipoproteins A-I, A-II, and B also rise across gestation, while HDL-cholesterol concentrations initially increase and then fall in the third trimester [9].
These changes in lipid metabolism help the mother and foetus to adapt. High triglyceride concentrations are thereafter used for maternal metabolic needs while sparing glucose for the foetus. The placental synthesis of steroid is facilitated by elevated LDL cholesterol levels. In short, the second trimester is characterised by accumulation of fat, which will be later consumed by the mother in the third trimester. Association of elevated lipid levels during normal pregnancy and development of atherosclerosis later in life has not been found out yet. But, it is seen that the fall in HDL levels in the third trimester of a normal pregnancy could be a potential risk factor for developing atherosclerosis [14]. Studies attempting to correlate the risk of coronary heart disease and number of pregnancies have produced contradictory results [15].
Leptin and adiponectin are members of the family of adipocytes, proteins secreted by the adipose tissue. Adipokines have an important role in the metabolism, inflammation, cardiovascular and endocrine system, mediating cross talk between insulin-sensitive tissues [16,17].
Leptin is a hormone which is secreted by adipose tissue in general and placenta during pregnancy. It plays an important role in fat metabolism by inhibiting hunger. During pregnancy, its serum concentration rises significantly, especially in second and third trimesters [18]. In spite of this rise in leptin levels; the appetite and food intake are still increased during pregnancy. It is because of resistance which develops to the central anorectic actions of leptin [19]. The ventromedial nucleus of the hypothalamus appears to be a key hypothalamic site involved in pregnancy-induced leptin resistance [20]. Leptin levels have been linked to pregnancy-specific pathologies such as gestational diabetes, preeclampsia, and intrauterine growth restriction. However, the functions of leptin in pregnant women, the foetus, and the placenta have not been determined; many observations suggest leptin is able to modulate insulin resistance [21–23].
Adiponectin is a protein produced by the maternal and foetal adipose tissue and plays a role in the modulation of glucose and lipid metabolism in insulin-sensitive tissues and the developing of gestational diabetes [23]. Adiponectin cord blood levels are significantly higher than maternal levels, with no correlation between the two compartments [24]. There appears to be a strong inverse correlation between maternal serum adiponectin levels and maternal glucose production [25]. It is seen that the maternal adiponectin levels are generally decreased during pregnancy, especially in third trimester of pregnancy when compared to non-gravid state [21]. This results in an increase in the glucose production by the liver which eventually helps foetal growth [26]. The reduction of maternal adiponectin levels found during pregnancy could be one of the pathway by which fat tissue stimulates the foetal growth through insulin sensitivity, thereby participating in the physiologic mechanism of insulin resistance.
The mean age of patients in our study was 24.87 years with a SD of 2.7 years. The minimum age was 18 years and the maximum age was 30 years.
We found a significant increase in the cholesterol, triglyceride, LDL-Cholesterol and VLDL-Cholesterol level in third trimester as compared to second trimester. We found HDL-Cholesterol levels decreased in third trimester when compared to second trimester.
In a case control by Giuseppe Lippi et al., a comprehensive lipid and lipoprotein profile was evaluated in 57 women at different gestational ages (20 in first, 20 in second and 17 in third trimesters, respectively). They concluded that all the lipid parameters were significantly modified particularly in second and third trimester when compared to non-pregnant females as well as when compared to the values in first trimester [27].
In a study done by Abdelhai AT, Jamil et al., a total of 115 pregnant women at different stages of pregnancy were included in the study, with 35 age-matched, healthy, non-pregnant women selected as control. They concluded that there was a significant decrease in high density lipoprotein cholesterol with increased low density lipoprotein concentrations. No significant difference was found in total cholesterol and triglycerides concentrations between pregnant women and non-pregnant controls [28].
A study was done on 160 women {120 pregnant women during normal gestation (40 women in each trimester) and 40 non-pregnant, healthy women as control} by Idonije O. Blessing et al., to evaluate the estimated serum concentrations of total cholesterol, high density lipoprotein, low density lipoprotein and triglycerides [29]. In this study, the concentrations of total cholesterol, high density lipoprotein and triglycerides during the first, second and third trimesters were significantly high (p< 0.05) as compared to that of the control subjects. However, the change in low density lipoprotein was not significantly high (p> 0.05) during the first trimester but was significantly high (p< 0.05) during the second and third trimester as compared to that of the control. The comparison between first, second and third trimesters showed that the serum concentrations of total cholesterol, triglycerides and low density lipoproteins during the 2nd and 3rd trimesters were significantly higher than they were in the 1st trimester. High density lipoprotein although not very significant in the 1st trimester followed the similar trend. Conclusively, increase in susceptibility to the development of coronary heart disease, arteriosclerosis, hypertension and other foetal/maternal diseases associated with dyslipidaemia in the subjects studied may be unlikely since the increase in LDL is accompanied by corresponding increase in the scavenging lipid- HDL. They concluded that lipid panel is of utmost importance and should be part of a routine investigation during pregnancy [29].
In a study conducted by Phuse SS et al., on 75 pregnant women between 24-35 years of age across each trimester of pregnancy against a control group of 70 non-pregnant women, estimated profile changes were observed. This study showed that the serum concentrations of total cholesterol, low density lipoprotein, very low density lipoprotein and triglycerides increased from trimester to trimester while that of high density lipoprotein decreased as compared to that of the control group. In short, lipid profile is variable during each trimester of a normal pregnancy [30]. The findings of our study correlate with the findings of various other international studies, which have been compared in the [Table/Fig-6].
Table representing few study results in comparison to the present study.
| Parameter{mg/dl} | Trimester | Present Study | Giuseppe Lippiet al., [27] | Abdelhai AT Jamilet al., [28] | Idonije O Blessinget al., [29] | Shital S Phuseet al., [30] |
|---|
| Serum Cholesterol | Second Trimester | 214.59 ± 18.16 | 243.0 ± 53 | 162.50 ± 24.01 | 191.4 ± 12.8 | 255.1 |
| Third Trimester | 242.64 ± 20.43 | 267.0 ± 30 | 170.10 ± 26.23 | 231.4 ± 9.1 | 270 |
| Serum Triglycerides | Second Trimester | 188.68 ± 20.87 | 151.0 ± 80 | 136.80 ± 58.3 | 217.5 ± 34.5 | 178.4 |
| Third Trimester | 216.78 ± 20.09 | 245.0 ± 73 | 175.90 ± 70.93 | 211.1 ± 26.3 | 198.8 |
| Serum HDL–Cholesterol | Second Trimester | 49.12 ± 6.14 | 83 ± 19 | 58.84 ± 19.27 | 44.4 ± 6.4 | 32.8 |
| Third Trimester | 43.06 ± 4.36 | 81 ± 17 | 38.09 ± 13.64 | 47.9 ± 3.8 | 27.6 |
| Serum LDL-Cholesterol | Second Trimester | 92.410±18.938 | 130 ± 46 | 67.94 ± 23.35 | 103.5 ± 16.2 | 170.9 |
| Third Trimester | 137.81 ± 13.45 | 136 ± 33 | 76.62 ± 26.95 | 141.2 ± 8.6 | 195.7 |
| Serum VLDL- Cholesterol | Second Trimester | 28.22 ± 7.66 | Not Described | Not Described | Not Described | 40.2 |
| Third Trimester | 36.27 ± 6.72 | Not Described | Not Described | Not Described | 45.7 |
Conclusion
Human gestation is associated with an “atherogenic” lipid profile which could act as a potential risk factor for pre-eclampsia and endothelial cell dysfunction, if further enhanced than the normal limits. This study helps in understanding baseline lipid parameters in the second and third trimester among pregnant women in India.
Total cholesterol, triglycerides, LDL-cholesterol, VLDL-cholesterol increased in both second and third trimester. The increase is more in third trimester, when compared to second. HDL-Cholesterol is decreased in third trimester when compared to second trimester.
As hypertriglyceridaemia is a risk factor for Pre-eclampsia, GDM and preterm, the estimation of lipid profile is highly recommended during pregnancy so as to institute prompt management strategies to prevent deleterious effect of hyperlipidaemia associated with pregnancy.