Introduction
In order to optimize desirable pharmacological responses to medications and minimizing the risk of adverse reactions to them, prediction and prevention of drug interplay is prime. Professor Bottiger stated: drug treatment has become more complicated with prescription of medicines with narrow therapeutic window, given concomitantly with many drugs, for longer periods, increasing the risk for drug interactions and also the patients are getting older. As a result the responsibility for drug treatment on doctors has increased [1]. The statement holds more relevance today, even after 39 years. The usage of drugs by patient is increasing continuously and so is the risk of drug interactions [2], which is an emerging concern for all fields of patient care.
Now, talking about the patient population of different age groups, Polypharmacy in geriatric dental population is the norm [3], due to increase in this population and presence of multiple disease states. Compound procedures involving restorative, periodontal, and implant over complete or partial dentures, is the treatment opted by many of these patients [4]. As a result, the need for local anaesthesia /vasoconstrictors, analgesics, anxiolytics, and antibiotics, on occasion could lead to adverse interactions with an array of drugs they are on. The intake of certain prescription medications, especially within the cardiovascular classes of drugs, among young to middle-aged adults, is on the rise. The unique physiology and anatomy of paediatric dental patients, make them vulnerable to drug interactions, mainly multiple central nervous system depressant drugs [5].
The aim of the present paper is to lay emphasis on those potential adverse drug interactions that are clinically relevant to the general dentist. The paper also provides an insight into the understanding of the general mechanisms behind such interactions, thereby aiding the clinician in identifying them before they occur.
Mechanisms of Drug Interactions: Alteration of one drug by either another drug or food items or environmental chemical agent is referred as an interaction [6]. Drugs interact in unique ways with each other, but in this paper, we have discussed about different interaction mechanism that is encountered repeatedly [Table/Fig-1].
Different drug interaction mechanisms.
| PHARMACODYNAMIC | PHARMACOKINETIC |
|---|
| SYNERGISTIC | ABSORPTION |
| ANTAGONISTIC | DISPLACEMENT |
| NEUROTRANSMITTER UPTAKE | BIOTRANSFORMATION |
| EXCRETION |
Pharmacokinetic Interactions: It involves the ability to alter the absorption, distribution, metabolism and excretion of one drug by another [6] (the so-called ADME interactions).
Drug absorption interactions: These involve drug administration via oral route involving more than two drugs or a drug along with food product. The effectiveness and blood levels of the drug are reduced due to the impairment of its ability caused by food product or other drug to cross mucous membrane in the stomach and intestine. Well known example of this type interaction to dental practitioners is chelation ability of systemic tetracycline and quinolone antibiotics to drugs and dairy products containing divalent and trivalent cations [7,8].
Drug displacement (protein-binding) interactions-[Table/Fig-2]: Drugs on absorption are rapidly distributed via circulation around the body. Some being completely dissolved in plasma water, but many others are transported in solution and rest are bound to plasma proteins, mainly albumins. The unbound portion of drug is pharmacologically active and free to interact with its receptors. Clinically significant interactions can occur when two extremely protein-bound drugs (usually >90%) are given concurrently and fight for receptor sites, due to the availability of finite number of protein-binding sites. So, one of the highly protein-bound drugs exhibit a rather low therapeutic index and is dislodged from plasma protein-binding sites by a so called extremely protein-bound displacer drug [9]. At supratherapeutic blood levels, the displaced drug, free in the plasma, likely results in a situation similar to an overdose of the displaced drug. NSAIDs, diazepam & chloral hydrate are probable displacer drugs used in dentistry.
Plasma protein binding characteristics of certain drugs and result of their displacement.
| DRUG | % PROTEIN BINDING | RESULT OF DISPLACEMENT |
|---|
| TOLBUTAMIDE, CHLORPROPAMIDE, GLYBURIDE, OTHER SULFONYLUREAS | 90-99 | HYPOGLYCAEMIA |
| WARFARIN [10] | 99 | BLEEDING |
| PHENYTOIN | 90 | CNS DEPRESSION, ATAXIA |
It involves the chemical alteration of the drug to either less lipid soluble or inactive form for easy excretion via kidney. The principal organ involved in the process is liver along with other tissues in the body, including the kidney, small intestine, bloodstream, and neuronal tissue. Drugs with a high first-pass effect, pre-hepatic metabolism in the small intestine is known to be an important site for metabolic drug interactions [11].
Well recognized biochemical target is the cytochrome P450 system for vast majority of drug metabolic interactions [12], a group of heme-containing enzymes embedded in the smooth endoplasmic reticulum of hepatocytes and enterocytes of liver and small intestine respectively. Cytochrome P450 isoenzymes known so far, are more than 30; CYP1A2, CYP2C9, CYP2D6, CYP2E1, and CYP3A4 being the significant ones. The isoform mostly concerned with greater number of metabolism of drugs and adverse interaction is CYP3A4. [13]. Two basic processes affecting metabolic enzymes in drug interactions are enzyme induction causing diminished drug effect and enzyme inhibition causing exaggerated effect due to overdose [Table/Fig-3].
CYP-450 enzyme substrates, inducers and inhibitors [14].
| CYP isoform | Substrates | Inducers | Inhibitors |
|---|
| CYP1A2 | Anti-Alzheimer: tacrine Antiasthmatic: theophyllineAntidepressants: fluvoxamine, imipramineAntipsychotics: clozapine, halperidol | Antibiotic: rifampinAnticonvulsant: carbamazepineFoods: char-grilled meatsRecreational drug: tobacco | Antibiotic: ciprofloxacin, erythromycin, ofloxacinAntidepressant: fluvoxamine |
| CYP2C9 | Angiotensin-2 receptor blockers: ibresartan, losartanAnticoagulant: warfarin Anticonvulsant: phenytoinHypoglycaemics: glipizide, glyburide, tolbutamideNon-steroidal anti-inflammatory drugs: diclofenac, ibuprofen, naproxen | Antibiotic: rifampinBarbiturates: phenobarbital, secobarbital | Antibiotic: metronidazole Antidepressants: fluvoxamine, paroxitene, sertralineAntifungal: fluconazole |
| CYP2D6 | Antidepressants: amitriptyline, desipramine, imipramine, paroxitene Antipsychotics: halperidol, risperidoneBeta-blockers: metoprolol, propranolol, timololNarcotic analgesics: codeine, hydrocodone, tramadol | Antibiotic: rifampinCorticosteroid: dexamethasone | Antidepressants: fluoxetine, paroxitene, sertraline Antiarrhythmic: amiodaroneH1 receptor blockers: hydroxyzine, promethazin |
| CYP2E1 | Alcohol: ethanolGeneral anaesthetics: enflurane, halothane, isoflurane, sevofluraneMuscle relaxer: chlorzoxazoneNon-narcotic analgesic: acetaminophen | Antibiotic: isoniazidRecreational drugs: ethanol, tobacco | Alcoholism rehabilitation agent: disulfiram |
| CYP3A4 | Antibiotics: clarithromycin, erythromycinAnticoagulant: warfarin Anticonvulsant: carbamazepineAntipsychotics: haloperidol, pimozideBenzodiazepines: alprazolam, diazepam, midazolam, triazolamCalcium channel blockers: amlodipine, diltiazem, felodipine, verapamil Cholesterol-lowering drugs: atorvastatin, cerivastatin*, lovastatin, simvastatin Corticosteroids: hydrocortisone, methylprednisoloneH1 receptor blockers: astemizole, terfenadineHIV protease inhibitor: idinavir, nelfinavir, ritonavir, saquinavirHormonal agents: estrogens, progestins Immunosuppressants: cyclosporine, tacrolimusLocal anaesthetic: lidocaine Prokinetic agent: cisapride | Antibiotic: rifampin Anticonvulsants: carbamazepine, phenytoinBarbiturates: phenobarbital, secobarbital Corticosteroids: dexamethasone, hydrocortisone, prednisolone, methylprednisolone Herbal remedy: St John’s wortHIV reverse transcriptase inhibitors: efavirenz, nevirapineHypoglycaemics: pioglitazone, troglitizone | Antibiotic: clarithromycin, erythromycin Antidepressants: fluvoxamine, nefazodoneAntifungals: clotrimazole, fluconazole, itraconazole, ketoconazoleCalcium channel blockers: diltiazem, verapamilFoods: Grapefruit juice, Seville oranges H2 receptor blocker: cimetidineHIV protease inhibitors: idinavir, nelfinivir, ritonavir, saquinavi |
With respect to drugs employed in dentistry, several benzodiazepines and narcotic analgesics come under substrate listings, while several commonly in use antimicrobial agents appear as enzyme inhibitors. When a cytochrome P450 substrate and a corresponding cytochrome P450-inducing or -inhibiting drug are administered on chronic basis, these types of interactions become most significant.
Drug excretion interactions-[Table/Fig-4]: The primary organ of drug elimination is kidney. The retention of supratherapeutic blood levels of the drug occurs due to the capability of one drug to weaken the renal elimination of another drug [Table/Fig-5].
Examples of interactions due to changes in renal transplant [6].
| Drug Affected | Interacting Drug | Result Of Interaction |
|---|
| CephalosporinsDapsoneMethotrexatePenicillinsQuinolones | Probenecid | Serum levels of drug affected raised; possibility of toxicity with some drugs |
| Methotrexate | Salicylates and some other NSAIDs | Methotrexate serum levels raised; serious methotrexate toxicity possible |
| Lithium | NSAIDs including ibuprofen, diclofenac, and naproxen. | High serum levels can lead to severe central nervous system and renal toxicity [15]. |
Types Of Pharmacodynamic interactions [6].
| Synergistic | Same pharmacological effect. | Ex-1- narcotic analgesic with alcohol2- NSAIDs with SSRIs |
| Antagonistic | Activity of drugs that are opposed to each other. | Ex-1- Naloxone against narcotic agonist2- ACE inhibitors with NSAIDs |
| Neuronal uptake | Drugs that block-• epinephrine’s activity at adrenergic receptors,• reuptake into the adrenergic neuronDegradation by catechol-O-methyltransferase [16,17]. | Ex-1- Tricyclic antidepressants-inhibit the reuptake2- MAOIs with epinephrine |
These interactions without altering drug’s concentration in tissue fluid revise its pharmacological effect [18]. Classification of these interactions is less easy than those of a pharmacokinetic type.
The rest of this review will condense about the significant drug interactions as they relate to dental practice, especially dealing with the drugs prescribed in common medical conditions [19] like hypertension, hypercholesterolaemia, CVD, haematological condition and depression.
Analgesic Agents: The use of analgesics is widespread, in dental or periodontal practice. Due to short-term duration of therapy and the relative low doses that are prescribed, serious adverse drug interactions involving them are rarely reported [11,20].
Non-Steroidal Anti-Inflammatory Drugs: With the ability to interfere with arachidonic acid metabolism & inhibit inflammatory process, the most commonly employed NSAIDs in dental practice includes of aspirin, ibuprofen, ketoprofen, naproxen, diclofenac, meloxicam and celecoxib. The recommended daily dosage is maximum of 2 g acetaminophen for alcoholics [Table/Fig-6,7]. If the dose remains in the therapeutic range there are little chances of toxicity [26]. The chances of enhanced gastrointestinal toxicity occur, if one consumes 3 or more drinks per day [20]. The recommendation is that consumption of aspirin and alcohol be separated by at least 12 hours [27]. The effect of interaction with antihypertensive drugs is observed when NSAIDs are taken for more than 5 days. Antihypertensive drugs like β-adrenergic blocking agents, ACE inhibitors and diuretics effects is dependent on prostaglandins [28]. By blocking the synthesis of the prostaglandins in the kidneys NSAIDs lower the antihypertensive effect [29–30].
Metabolism of paracetamol and ethanol [14].
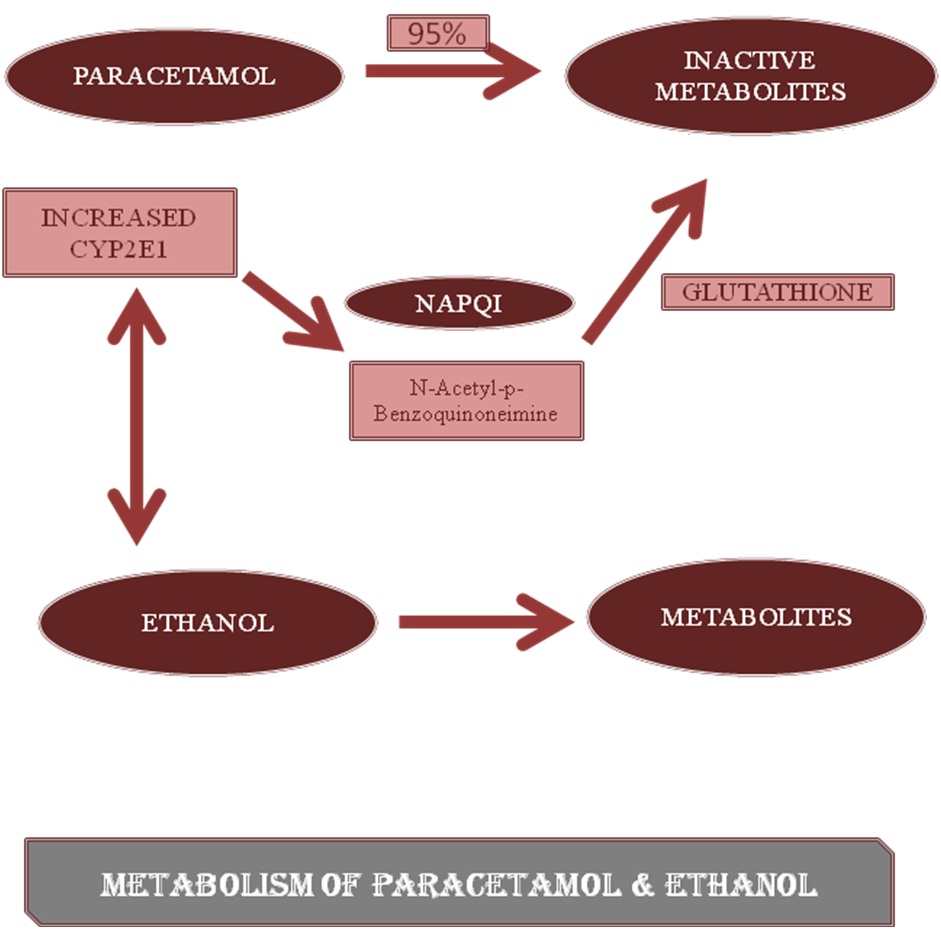
Effects of various NSAIDs on interaction with alcohol.
| ALCOHOL | Acetaminophen | Increase the levels of N-acetyl-para-benzoquinonemine (highly reactive metabolite) [21,22]. [Table/Fig-4] |
| Aspirin | Increases the risk of fecal blood loss associated with gastrointestinal erosions and ulcers [23] |
| Ibuprofen, Ketoprofen Naproxen | • Gastrointestinal adverse effects- upper git bleeding [24]• Renal toxicity [25] |
When NSAIDs are administered concomitantly with high dose of methotrexate [31], decrease in prostaglandin-dependent renal perfusion and consequent elimination of methotrexate results. The combination of NSAIDs with Selective Serotonin Reuptake Inhibitors must be avoided where ever possible. According to a meta-analysis, the risk of upper gastrointestinal haemorrhage is serious, due to SSRIs impaired vasoconstrictive potential and NSAIDs risk of gastric damage [32]. Impairment of thromboprophylactic action of aspirin [33] is seen when administered with other NSAIDs. Theoretically, when both aspirin and a NSAIDs are involved all at once, drugs like ibuprofen could vie with aspirin for the cyclooxygenase-1 binding site in the platelet [34]. As a result the synthesis of thrombaxane A2 may resume after subsequent removal of NSAID.
Narcotic Analgesic Agents: Patients managed with opioid analgesics following treatment to manage post-procedural pain, are at risk of serious drug interactions. The metabolism of most of the opioid analgesics is by CYP2D6. In the case of codeine, the active demethylated metabolite morphine & the parent molecule, in case of tramadol, appears to possess analgesic activity by enhancing noradrenergic and serotonergic activity in the central nervous system [35]. Their analgesic effect is eradicated, on administration of antiarrhythymic agent quinidine, a known CYP2D6 inhibitor [36].
Normeperidine effects are worsened due to deactivation of neurotoxic meperidine metabolite by monoamine oxidase inhibitors. Life-threatening episodes have been reported in monoamine oxidase inhibitor consumers with therapeutic doses of meperidine. Similarly, other opioids in the phenylpiperidine series, including tramadol and propoxyphene, have their own intrinsic serotonergic activity, which may bring into being a serotonin-like syndrome when taken at the same time with monoamine oxidase inhibitors [37].
Behaviour Modifying Agents [Table/Fig-8]: Orally administered behaviour modifying agents, for patient relaxation and release of anxiety, are valuable additions to a dentist’s pain control armamentarium. Signs of excessive central nervous system depression: lethargy, prolonged sedation, loss of consciousness, and/or respiratory depression, is the most significant systemic adverse drug reactions related with these agents. CYP3A4 isoenzymes are inhibited by drugs like calcium-channel blockers verapamil and diltiazem [38], cimetidine [39] and protease inhibitors [40]. Due to its inhibition the metabolism of benzodiazepine like triazolam, oral midazolam, alprazolam is altered resulting in their 2-3 fold increase in blood. Antimicrobials like erythromycin, clarithromycin, ciprofloxacin, and the azole antifungals are potential inhibitors of enzymes needed for the triazolam and oral midazolam’s metabolism [41]. Hepatic enzymes required for the oxidative metabolism of certain benzodiazepines: alprazolam, triazolam, and midazolam; are induced by rifampin [42] and carbamazepine [6].
Examples of behaviour modifying agents interacting with other drugs.
| Benzodiazepines | Barbiturate | Chloral Hydrate |
|---|
| Increase in the rate of metabolism (eg.,rifampin, carbamazepine)Decrease in the rate of metabolism (eg., cimetidine, erythromycin, indinavir) | Ex: Warfarin, Phenobarbital | Ex: Warfarin, Alcohol |
Barbiturates are less specific with dental procedures, compared to the currently available benzodiazepines. In order to maintain therapeutic prothrombin times, an increment of 30% in warfarin doses is needed when administered in patients on chronic barbiturate therapy. Chloral Hydrate is the most commonly used oral sedative in paediatric dentistry. It is found to be involved in a variety of drug interactions. Combination with alcohol results in supraadditive interaction due to alteration of alcohol metabolism.
Local Anaesthetics [Table/Fig-9]: They are all central nervous system depressants and function by inhibiting neuronal functions [43]. The greatest concern to general practitioners and specialists, are the drug interactions that supplement the severity of central nervous system depression. For adults, the maximum number of 1.8-ml cartridges that can be used of 2% lidocaine with 1:100,000 epinephrine and of 3% mepivacaine are 14 and 7 respectively [44]. A summation drug interaction predicts that the maximum dose of grouping of these agents would be seen with seven cartridges of 2% lidocaine with epinephrine and 3.5 cartridges of mepivacaine.
List of various drugs interacting with local anaesthesia and vasoconstrictors.
| Benzodiazepines | Barbiturate |
|---|
| LOCAL ANAESTHESIA | • Summation interactions (Ex: Lidocaine & Bupivacaine) |
| • Amide (Ex: Lidocaine with Cimetidine and Propranolol) |
| • Opioid (Ex: Mepivacaine with Meperidine) |
| • Ester (Ex: Procaine with sulfamethoxazole) |
| • Strong oxidizing drugs (Ex: Prilocaine with Dapsone) |
| VASOCONSTRICTORS | • Adrenergic neuronal blocking agents |
| • Digitalis glycosides |
| • Cocaine |
| • Tricyclic antidepressants |
| • β-adrenergic blocking agents |
| • Attention deficit hyperactivity disorder drugs |
| • Comt inhibitors |
Amide local anaesthetics metabolism is inhibited by certain drugs. Cimetidine (H2-histamine antagonist) a known inhibitor of hepatic oxidative enzymes is required for the biotransformation of many drugs including lidocaine. A 50% increase in blood concentration following steady-state infusion occurs, thereby slowing the elimination of lidocaine [45]. Reduction in clearance of lidocaine occurs by β-adrenergic blocker propranol, by 40% [46]. When treating paediatric dental patients, systemic depressant effects of local anaesthetics are most apparent due to possible interactions with other central nervous system depressants. One-third the dose of a 150-lb (68-kg) adult for a child of 50-lb (23-kg) is the maximum recommended safe dose. Opioids usage has been correlated with local anaesthetic toxicity reactions [47]. The mechanism for this interaction is probably many-sided. In part, when excessive doses are administered, synthetic opioids, such as meperidine, have convulsant properties [48]. There is decrease in protein binding of local anaesthetics on opioid administration, resulting in distribution of more unbound drug to the central nervous system.
Theoretically, there may be reduction in sulphonamide antibacterial activity following use of ester local anaesthetics [49]. Sulphonamide inhibition is antagonized by an increase in concentration of p-amino benzoic acid available to bacteria due to metabolism of ester local anaesthetics. Development of drug-induced methemoglobinaemia is associated when excessive doses of dental anaesthetics like prilocaine and benzocaine (and rarely lidocaine and articaine) is administered. Methemoglobin production results due to concomitant drug therapy with various nitrite preparations, antimicrobials dapsone and sulphonamides and the analgesic phenacetin [50]. It is recommended that local anaesthetic dose be calculated carefully and that the weight-based maximum safe dosage recommendations not exceeded.
Vasoconstrictors [Table/Fig-9]: Of all the drugs prescribed in dentistry, vasoconstrictors are of most worry due to potential adverse drug interactions with them. Non- selective β-adrenergic blocking agents on interaction with epinephrine block the β2 vasodilatory effects of epinephrine, allowing the α1 vasoconstrictive effects to function unopposed. Case reports of patients on non-selective β-adrenergic blockers have demonstrated serious sequel injections of local anaesthetic volumes equivalent to two cartridges of 2% lidocaine plus 1:50,000 epinephrine [51]. It is recommended that individuals on non-selective β-adrenergic blocking agents receive an initial test dose of no more than 0.04 mg epinephrine or 0.2 mg levonordefrin (two cartridges of a 1:100,000 epinephrine solution or two cartridges of a 1:20,000 levonordefrin solution) and then be monitored for increase in blood pressure before additional local anaesthetic is administered [52].
Tricyclic antidepressant’s potential to block the non-adrenergic reuptake pump, the accumulation of epinephrine and levonordefrin in the vicinity of postsynaptic α- and β-adrenergic receptors could result, leading to enhanced cardiovascular activity. Epinephrine dosages should not exceed 0.054 mg and levonordefrin and norepinephrine should be avoided. Cocaine possesses tricyclic antidepressant-like activity and may also enhance adrenergic neurotransmitter release and postsynaptic responses to epinephrine-like drugs. After the last dose of cocaine, for at least 48 hours the use of vasoconstrictors should be suspended [52]. Norepinephrine reuptake inhibitor atomoxetine and amphetamine or amphetamine-like stimulants (Attention Deficit Hyperactivity Disorder Drugs) are the commonly employed drugs [53], resulting in increased release of norepinephrine and other catecholamines and also blocking their reuptake [54]. These dosage of epinephrine (0.04–0.054 mg) or levonordefrin (0.2 mg) can be used in children and adults with normal blood pressures and heart rates.
Recently introduced drugs as adjuncts to levodopa/carbidopa, in the management of Parkinson’s disease are tolcapone and entacapone (Catechol-O-Methyltransferase Inhibitors). They reversibly block catechol-O-methyltransferase, inhibiting levodopa inactivation in the periphery [55]. Inactivation of epinephrine and levonordefrin enclosed in a local anaesthetic solution is also inhibited by them. It has been recommended that no more than the equivalent of one cartridge of lidocaine with 1:100,000 epinephrine be administered initially and to monitor the patient’s blood pressure and heart rate before administering any additional local anaesthetic with vasoconstrictor [55]. Adrenergic neuronal-blocking agents, guanethidine and guanadrel both deplete and inhibit the release of norepinephrine from adrenergic nerve terminals. Subjects pretreated with guanethidine that received norepinephrine infusions, showed significant increase in press or response with more frequent cardiac arrhythmias [56]. Recommended epinephrine dose not exceeding 0.054 mg and careful aspirating technique is advised. For population on digitalis glycosides, cautious use of dental vasoconstrictors is recommended, due to induction of additive dysrhythmogenic activity.
Antibiotic/Antifungal Agents: Duration of drug therapy with antimicrobial agents is more long-lasting than other drug classes used in dental practice, which increases the hazard of adverse drug interactions compared to other drug classes. Possibility of adverse drug interactions in dentistry is increased due to four commonly employed antibiotics (clarithromycin, erythromycin, ciprofloxacin, and metronidazole), which are potent inhibitors of various cytochrome P450 isoforms. Ciprofloxacin and erythromycin are CYP1A2 isoenzyme inhibitors, which reduce the biotransformation and raise the blood levels of CYP1A2 substrate drugs. Example of such drugs are (Fluvoxamine, imipramine); (Theophylline); (Clozapine, Halperidol) and (Tacrine) [57]. Most clinically relevant interactions for practicing dentists is the ability of metronidazole & fluconazole to significantly increase the blood concentrations and half- life of the anticoagulant warfarin [58] & antiepileptic phenytoin [59], which are CYP2C9 substrates. CYP3A4 metabolizes the greatest number of drug substrates and correspondingly is involved in the greatest number of possible metabolic drug interactions, of all the cytochrome P450 isoenzymes {[Table/Fig-10] lists such interactions}.
Antibiotics (erythromycin, clarithromycin and azole antifungal) drug interaction with some cyp3a4 substrates [14].
| CYP3A4 substrates | Potential interaction |
|---|
| Astemizole, Cisapride, Pimozide, Terfenadine | Cardiac QT interval prolongation and torsades de pointes ve |
| Atorvastatin, Cerivastatin, Lovastatin, Simvastatin | Diffuse myalgias, rhabdomyolysis, and renal failure |
| Felodipine, Nifedipine And Possibly Other Calcium Channel Blockers | Antihypertensive Effect |
| Cyclosporine, Tacrolimus | Immunosuppression and Nephrotoxicity |
| Warfarin | Increased prothrombin times, international normalized ratios, and an increased risk of serious bleeding |
| Carbamazepine | Risk of ataxia, vertigo, drowsiness, and confusion |
| Alprazolam, Diazepam, Midazolam, Triazolam | Excessive and prolonged sedation |
Metronidazole, like disulfiram, in the ethanol degradation pathway inhibits the enzyme acetaldehyde dehydrogenase, resulting in an accumulation of acetaldehyde in the bloodstream. Avoidance of alcohol consumption is needed during metronidazole therapy and for at least 3 days after that. Due to low therapeutic index of digoxin, antibiotics like clarithromycin, erythromycin, and azithromycin should be avoided in patients on digoxin therapy. Its the ability of these antibiotics to inhibit P-glycoprotein, contributing to the swift increase in digoxin blood levels in patients ensuing in classic digitalis toxicity [60]. One of the most debated interactions is the reported capability of commonly prescribed antibiotic agents to lower blood levels and efficacy of oral contraceptive agents [61]. Oral contraceptive failure reports have appeared in literature, following therapy with certain antibiotics like tetracyclines, penicillins, erythromycin, metronidazole and cephalosporins [62]. Plausible mechanism is that the enterohepatic recirculation of the estrogen steroid component of the pill is inhibited by common antibiotics [62].
Conclusion
Drug interactions are an avoidable cause of patient harm. With the continued introduction of new therapeutic classes of drugs, the number of potential adverse drug interactions will continue to grow. Drug interactions should be considered both in the differential diagnosis of symptoms and when prescription changes are made. In order to prevent drug interactions, the sound place to start is with patient’s current medical history and medication intake. Vigilance regarding recognition and prevention of such interactions is needed by dental clinician.
[1]. Bottiger Y, Drug–drug interactions today–from research to clinical practiceJ Intern Med 2010 268(6):511 [Google Scholar]
[2]. Haider SI, Johnell K, Thorslund M, Fastbom J, Trends in polypharmacy and potential drug-drug interactions across educational groups in elderly patients in Sweden for the period 1992-2002Int J Clin Pharmacol Ther 2007 45:643-53. [Google Scholar]
[3]. Heft MW, Mariotti A, Geriatric pharmacology. In: Yagiela JA, Dowd FJ, Neidle EA editorsPharmacology and Therapeutics in Dentistry 2004 5th ednOxfordElsevier Mosby:849-856. [Google Scholar]
[4]. Davis BK, Dental aesthetics and the aging patientFacial Plast Surg 2006 22:154-60. [Google Scholar]
[5]. Webb MD, Moore PA, Sedation for paediatric dental patientsDent Clin N Am 2002 46:803-14. [Google Scholar]
[6]. Stockley IH, Stockley’s Drug Interactions 2008 8th ednLondonPharmaceutical Press [Google Scholar]
[7]. Nix DE, Watson WA, Lener ME, Frost RW, Krol G, Goldstein H, Effects of aluminium and magnesium antacids and ranitidine on the absorption of ciprofloxacinClin Pharmacol Ther 1989 46:700-05. [Google Scholar]
[8]. Leyden JJ, Absorption of minocycline hydrochloride and tetracycline hydrochloride. Effect of food, milk, and ironAm Acad Dermatol 1985 12:308-12. [Google Scholar]
[9]. Christensen H, Baker M, Tucker GT, Rostami-Hodjegan A, Prediction of plasma protein binding displacement and its implication for quantitative assessment of metabolic drug–drug interactions from in vitro dataJ Pharm Sci 2006 95:2778-87. [Google Scholar]
[10]. Seymour RA, Drug interactions in dentistryDent Update 2009 36:458-70. [Google Scholar]
[11]. Moore PA, Gage TW, Hersh EV, Yagiela JA, Haas DA, Adverse drug interactions in dental practice: professional and educational implicationsJ Am Dent Assoc 1999 130:47-54. [Google Scholar]
[12]. Hersh EV, Moore PA, Drug interactions in dentistry: the importance of knowing your CYPsJ Am Dent Assoc 2004 135:298-311. [Google Scholar]
[13]. Venkatakrishan K, Van Molt LL, Greenblatt DJ, Effect of the antifungal agents on oxidative drug metabolismClin Pharmacokinet 2000 38:111-80. [Google Scholar]
[14]. Hersh EV, Moore PA, Adverse drug interactions in dentistryPeriodontology. 2000 2008 46:109-42. [Google Scholar]
[15]. Ragheb M, The clinical significance of lithium–non-steroidal anti-inflammatory drug interactionsJ Clin Psychopharmacol 1990 10:350-54. [Google Scholar]
[16]. Naftalin LW, Yagiela JA, Vasoconstrictors: indications and precautionsDent Clin North Am 2002 46:733-46. [Google Scholar]
[17]. Yagiela JA, Adverse drug interactions in dental practice: interactions associated with vasoconstrictorsJ Am Dent Assoc 1999 130:701-09. [Google Scholar]
[18]. Rang HP, Dale MM, Ritter JR, Flower RJ, Henderson G, Rang and Dale’s pharmacology 2012 6th edUKChurchill Livingstone [Google Scholar]
[19]. Dawoud BES, Roberts A, Yates JM, Drug interactions in general dental practice-considerations for the dental practitionerBritish Dental Journal 2014 216(1):15-23. [Google Scholar]
[20]. Hersh EV, Moore PA, Ross GL, Over-the-counter analgesics and antipyretics: a critical assessmentClin Ther 2000 22:500-48. [Google Scholar]
[21]. Whitcomb DC, Block GD, Association of acetaminophen hepatotoxicity with fasting and ethanol useJAMA 1994 272:1845-50. [Google Scholar]
[22]. Tanaka E, Yamazaki K, Misawa S, Update: the clinical importance of acetaminophen hepatotoxicity in non-alcoholic and alcoholic subjectsJ Clin Pharm Ther 2000 25:325-32. [Google Scholar]
[23]. Goulston K, Cooke AR, Alcohol, aspirin and gastrointestinal bleedingBr Med J 1977 2:1532-36. [Google Scholar]
[24]. Neutel CI, Appel WC, The effect of alcohol abuse on the risk of NSAID-related gastrointestinal eventsAnn Epidemiol 2000 10:246-50. [Google Scholar]
[25]. Johnson GR, Wen SF, Syndrome of flank pain and acute renal failure after binge drinking and nonsteroidal anti-inflammatory drug ingestionJ Am Soc Nephrol 1995 5:1647-52. [Google Scholar]
[26]. Dart RC, Kuffner EK, Rumack BH, Treatment of pain of fever with paracetamol (acetaminophen) in the alcoholic patient: a systemic reviewAm J Ther 2000 7:123-34. [Google Scholar]
[27]. Haas DA, Adverse drug interactions in dental practice: interactions associated with analgesicsJ Am Dent Assoc 1999 130:397-407. [Google Scholar]
[28]. Houston MC, Nonsteroidal anti-inflammatory drugs and antihypertensivesAm J Med 1991 90(Suppl. 5A):42-78. [Google Scholar]
[29]. Hersh EV, Lally ET, Moore PA, Update of cyclooxygenase inhibitors: has a third COX isoform entered the fray?Curr Med Res Opin 2005 21:1217-26. [Google Scholar]
[30]. White WB, Cardiovascular risk, hypertension and NSAIDsCurr Rheumatol Rep 2007 9:36-43. [Google Scholar]
[31]. Frenia ML, Long KS, Methotrexate and nonsteroidal anti- inflammatory drug interactionsAnn Pharmacother 1992 26:234-37. [Google Scholar]
[32]. Loke YK, Trivedi AN, Singh S, Meta-analysis: gastrointestinal bleeding due to interaction between selective 5-hydroxytrptamine uptake inhibitors and non-steroidal anti-inflammatory drugsAliment Pharmacol Ther 2008 27:31-40. [Google Scholar]
[33]. Schuijt MP, Huntjens-Fleuren HW, de Metz M, Vollaard EJ, The interaction of ibuprofen and diclofenac with aspirin in healthy volunteersBr J Pharmacol 2009 157:931-34. [Google Scholar]
[34]. Catella-Lawson F, Reilly MP, Kapoor SC, Cucchiara AJ, DeMarco S, Tournier B, Cyclooxygenase inhibitors and the antiplatelet effects of aspirinN Engl J Med 2001 345:1809-17. [Google Scholar]
[35]. Enggaard TP, Poulsen L, Arendt-Nielsen L, Brosen K, Ossig J, Sindrup SH, The analgesic effect of tramadol after intravenous injection in healthy volunteers in relation to CYP2D6Anaesth Analg 2006 102:146-50. [Google Scholar]
[36]. Garrido MJ, Sayar O, Segura C, Rapado J, Dios-Vieitez MC, Renedo MJ, Pharmacokinetic / pharmacodynamic modeling of the antinociceptive effects of (+)-tramadol in the rat: role of cytochrome P450 2D activityJ Pharmacol Exp Ther 2003 305:710-18. [Google Scholar]
[37]. Gillman PK, Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicityBr J Anaesth 2005 95:434-41. [Google Scholar]
[38]. Varhe A, Olkkola KT, Neuvonen PJ, Diltiazem enhances the effect of triazolam by inhibiting its metabolismClin Pharm Ther 1996 59:369-75. [Google Scholar]
[39]. Sanders LD, Whitehead C, Gildersleve CD, Rosen M, Robinson JO, Interaction of H2-receptor antagonists and benzodiazepine sedationAnaesthesia 1993 48:286-92. [Google Scholar]
[40]. Heylen R, Miller R, Medications commonly used in the treatment of adult HIV positive patients: Part 2Genitourin Med 1997 73:5-11. [Google Scholar]
[41]. Varhe A, Olkkola KT, Neuvonen PJ, Oral triazolam is potentially hazardous to patients receiving systemic antimycotics ketoconazole or itraconazoleClin Pharm Ther 1994 56:601-07. [Google Scholar]
[42]. Backman JT, Olkola KT, Neuvonen PJ, Rifampin drastically reduces plasma concentrations and effects of oral midazolamClin Pharm Ther 1996 59:7-13. [Google Scholar]
[43]. Yagiela JA, Local anaesthetics. In: Yagiela JA, Dowd FJ, Neidle EA editorsPharmacology and Therapeutics for Dentistry 2004 5th ednSt LouisMosby-Year Book:251-270. [Google Scholar]
[44]. Ciancio SG, ADA Guide to Dental Therapeutics 2003 3rd ednChicagoADA Publishing Co [Google Scholar]
[45]. Freely J, Wilkson GR, McAllister CB, Wood AJ, Increased toxicity and reduced clearance of lidocaine by cimetidineAnn Int Med 1982 96:592-94. [Google Scholar]
[46]. Bax ND, Tucker GT, Lennard MS, Woods HF, The impairment of lignocaine clearance by propranolol – major contribution from enzyme inhibitionBr J Clin Pharmacol 1985 19:597-603. [Google Scholar]
[47]. Moore PA, Goodson JM, Risk appraisal of narcotic sedation for childrenAnaesth Prog 1985 32:129-39. [Google Scholar]
[48]. Gebhart GF, Opioid analgesics and antagonists. In: Yagiela JA, Dowd FJ, Neidle EA editorsPharmacology and Therapeutics in Dentistry 2004 5th ednOxfordElsevier Mosby:315-330. [Google Scholar]
[49]. Woods DD, The relation of p-aminobenzoic acid to the mechanism of action of sulfanilamideBr J Exp Pathol 1940 21(2):74-90. [Google Scholar]
[50]. Hersh EV, Stoopler E, Secreto SA, DeRossi SS, A study of benzocaine gel dosing for toothacheJ Clin Dent 2005 16:103-08. [Google Scholar]
[51]. Centeno RF, Yu YL, The propranolol–epinephrine interaction revisited: a serious and potentially catastrophic adverse drug interaction in facial plastic surgeryPlast Reconstr Surg 2003 111:944-45. [Google Scholar]
[52]. Naftalin LW, Yagiela JA, Vasoconstrictors: indications and precautionsDent Clin North Am 2002 46:733-46. [Google Scholar]
[53]. Moore PA, Hersh EV, Common medications prescribed for adolescent dental patientsDent Clin North Am 2006 50:139-49. [Google Scholar]
[54]. Nissen SE, ADHD drugs and cardiovascular riskN Engl J Med 2006 354:1445-58. [Google Scholar]
[55]. Rosenberger M, Yagiela J, Drug interactions: COMT inhibitorsJ Mass Dent Soc 2001 50:44-46. [Google Scholar]
[56]. Muelheims GH, Entrup RW, Paiewonsky D, Mierzwiak DS, Increased sensitivity of the heart to catecholamine-induced arrhythmias following guanethidineClin Pharmacol Ther 1965 6:757-62. [Google Scholar]
[57]. Bentue-Ferrer D, Tribut O, Polard E, Allain H, Clinically significant drug interactions with cholinesterase inhibitors: a guide for neurologistsCNS Drugs 2003 17:947-63. [Google Scholar]
[58]. Kazmier FJ, A significant interaction between metronidazole and warfarinMayo Clin Proc 1976 51:782-84. [Google Scholar]
[59]. Blum RA, Wilton JH, Hilligoss DM, Gardner MJ, Henry EB, Harrison NJ, Effect of fluconazole on the disposition of phenytoinClin Pharmacol Ther 1991 49:420-25. [Google Scholar]
[60]. Rengelshausen J, Goggelmann C, Burhenne J, Riedel KD, Ludwig J, Weiss J, Contribution of increased oral bioavailability and reduced nonglomerular renal clearance of digoxin to the digoxin– clarithromycin interactionBr J Clin Pharmacol 2003 56:32-38. [Google Scholar]
[61]. DeRossi SS, Hersh EV, Antibiotics and oral contraceptivesDent Clin N Am 2002 46:653-64. [Google Scholar]
[62]. Bainton R, Interaction between antibiotic therapy and contraceptive medicationOral Surg 1986 61:453-55. [Google Scholar]