Despite significant developments in the diagnostic modalities for immune injury in renal allograft, biopsy still remains the gold standard for evaluation of Graft Dysfunction (GD) [1,2]. It provides valuable insights into pathogenesis of early and late allograft injury and is indispensable for the diagnosis of renal transplant (RT) rejection and its clinical management [2]. On an average, biopsy findings change the clinical diagnosis in 36% and therapeutic management in 59% of cases [3]. Apart from immunological injury which is of utmost significance, the other causes of GD are acute ischemia reperfusion injury or acute tubular necrosis (ATN), drug toxicity, infections, obstruction/reflux, renal artery stenosis, de novo glomerular diseases, recurrent primary diseases and auto/alloantibody mediated diseases or related to technical issues [3].
We evaluated renal allograft biopsies to determine the causes of early (0-6 months) and late (> 6 months post-transplantation) GD. We studied the frequency of peritubular capillary (PTC) C4d positivity by immunohistochemistry and correlated with microvascular inflammation in Antibody Mediated Rejection (AMR).
Materials and Methods
This was a single center prospective study on diagnostic i.e., “clinically indicated” RT biopsies performed over a period of 2 months (between January,’15 and March,’15).
The graft biopsy specimens were processed for light microscopy and C4d immunohistochemistry (IHC) as per standard protocols. For light microscopy, 3μm thick sections were stained with Haematoxylin and Eosin, Gomori’s trichrome, Periodic Acid Schiff and Jones silver methaneamine stains. IHC was performed on 3μm thick paraffin sections using “NovolinkTM Polymer Detection System” (Leica Biosystems) with rabbit anti-human C4d monoclonal antibody (clone SP91, Spring Bioscience) and NovolinkTM Polymer Anti-rabbit Poly-HRP-IgG. Patient-donor demographics including immunosuppression and monitoring along with serum creatinine (SCr) levels were collected from patient case files. Optimal biopsy was defined as a specimen with at least 10 non-sclerotic glomeruli and 2 arteries; a marginal biopsy having 7 to 9 glomeruli and 1 artery; a minimally acceptable biopsy having 7 glomeruli and 1 artery [1,4]. Specimens with < 7 glomeruli or no arteries or with only medulla were considered as non- diagnostic.
Histological categories were classified as per Banff’13 modified update diagnostic categories for renal allograft biopsies into six categories; normal (category-1), AMR (category-2), borderline T-cell mediated rejection (category-3), T-cell-mediated rejection (TCR) (category-4), interstitial fibrosis and tubular atrophy (IFTA) (category-5) and others: changes not due to rejection/ non-rejection causes (category-6) [3,5,6]. “Revised Banff,’13 criteria for classification of AMR which includes C4d-Negative AMR was used for AMR diagnosis [6]. The Banff scoring system (scores ranging from 0-3) was used for the grading of acute and chronic changes occurring in the interstitium, tubules, glomeruli, arteries and arterioles [3,5,6]. C4d staining of the PTC was graded as C4d0-negative, C4d1- minimal: (1-10%), C4d2-focal: (10-50%), and C4d3-diffuse > (50%) PTCs [7]. PTC C4d deposition was considered positive if grade was > C4d0 and negative if C4d0 [6].
All the cases were under standard immunosuppression protocol comprised of prednisolone (10-20 mg/day), Tacrolimus (0.03- 0.05 mg/Kg/day) and /or mycofenolate sodium (360 mg, three or four times a day).
Statistical Analysis
The frequency of each category of renal disease was computed. All continuous parameters were expressed as mean and standard deviation, and, all qualitative variables as proportion. Fisher-Exact test was used to compare C4d score with degree of microvascular inflammation. Data was analysed using Microsoft Excel. The p < 0.05 was considered as statistically significant. The sensitivity of C4d in detecting AMR was calculated as a/a+b {a = true positive (No. of C4d positive AMR), b = false negative (No. of C4d negative AMR)}.
Results
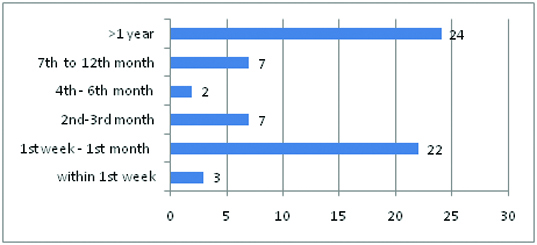
A total of 67 biopsies from 67 patients were analysed, of which two were excluded as the specimens were inadequate. Of the remaining 65 cases included in the study, 56 (86.2%) were optimal, seven (10.7%) were marginal and two (3.1%) were minimally acceptable biopsies. There were 57 (87.7%) males and 8 (12.3%) females. Mean recipient age was 34.7 for males (age range 13- 58 years) and 34 years for females (age range 23- 49 years). Clinical indications of allograft biopsy were rejection in 38 (58.5%), asymptomatic increase in serum creatinine (SCr) levels (of 20% above the baseline) in 17 (26.2%), ATN in 5 (7.7%), recurrent glomerulonephritis in 2 (3.1%), delayed graft function in 2 (3.1%) and proteinuria in 1 (1.5%). The time of allograft biopsies ranged from 5 days to 8 years post transplantation. 52.3% (34/65) of the biopsies were performed in the first 6 months and 47.7% (31/65) were performed after 6 months, post-transplantation [Table/Fig-1]. The histological findings are shown in [Table/Fig-2].
Timing of allograft biopsies post transplantation. x axis indicates time of biopsy, y indicates No. of cases..
Histological findings in renal allograft biopsies.
| Banff diagnostic category | Number of cases | Percentage |
|---|
| Normal (category 1) | 7 | 10.8 |
| AMR (category 2) | 10 | 15.4 |
| Borderline T-cell rejection (category 3) | 2 | 3.1 |
| AMR + Borderline T-cell rejection (categories 2+3) | 5 | 7.7 |
| (TCR) (category 4) | 3 | 4.6 |
| AMR+TCR (categories 2+4) | 9 | 13.7 |
| Others: changes not due to rejection (category 6). | 18 | 27.8 |
| AMR + Others (categories 2+6) | 5 | 7.7 |
| AMR +TCR +others (categories 2+4+6) | 6 | 9.2 |
| Total | 65 | 100.0 |
AMR- Antibody mediated rejection, TCR- T-cell-mediated rejection.
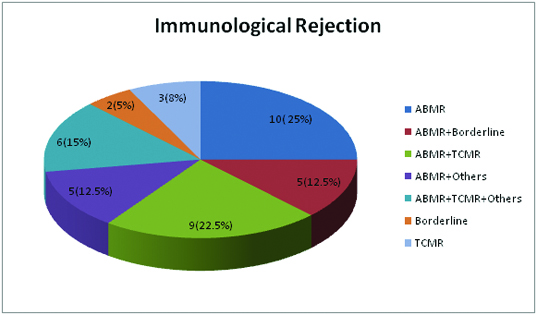
The incidence of immune injuries was observed in 40 (61.5%) biopsies and predominant immune injury was AMR observed in 35 (87.5%) biopsies [Table/Fig-3].
Types of rejection with or without superimposed non-rejection causes.
ABMR- Antibody mediated rejection, Others- non-rejection causes, TCMR- T-cell-mediated rejection. The 2 cases of borderline rejection were amenable to steroid therapy and hence were considered to be earliest evidence of immunological activity.
Non-rejection causes were observed in 29 (44.6%) biopsies [Table/Fig-4] with Calcineurin Inhibitor Toxicity (CNI Toxicity) (12/29) being the commonest followed by ATN (5/29).
Various histological diagnosis in Category 6 with or without superimposed rejection.
| Histological diagnosis | Non-rejection causes | Non-rejection causes superimposed on AMR | Non-rejection causes superimposed on AMR&TCR | Total |
|---|
| CNI Toxicity | 5 | 2 | 3 | 10 |
| CNI Toxicity + BKVN | 2 | - | - | 2 |
| BKVN | 1 | 1 | - | 2 |
| Acute pyelonephritis | 1 | - | - | 1 |
| ATN | 3 | 1 | 1 | 5 |
| Arteriolar hyalinosis (donor related) | 1 | - | - | 1 |
| Post transplant TMA | 3 | - | - | 3 |
| De novo crescentic Glomerulonephritis | - | - | 1 | 1 |
| De novo Collapsing glomerulopathy. | - | 1 | - | 1 |
| De novo FSGS | - | - | 1 | 1 |
| De novo oxalosis | 2 | - | - | 2 |
| Total | 18 | 5 | 6 | 29 |
AMR- Antibody mediated rejection, ATN- Acute tubular injury, BKVN- BK viral nephropathy, CNI- Calcineurin Inhibitor, FSGS- Focal segmental glomerulosclerosis, TCR- T-cell-mediated rejection, TMA- Thrombotic microangiopathy.
The histological findings corresponding to the timing of biopsies is depicted in [Table/Fig-5] and the histological diagnosis in category 6, with or without superimposed rejection, based on timing of biopsies is shown in [Table/Fig-6].
Histological findings corresponding to the timing of biopsies.
| Biopsy timing | Normal | AMR | Borderline T-cell rejection | AMR+ Borderline T-cell rejection | TCR | AMR + TCR | Others | AMR + Others | AMR + TCR + Others | Total |
|---|
| within 1st week | - | 1 | - | - | - | - | 2 | - | - | 3 |
| 1st week – 6th month | 5 | 7 | 2 | 5 | 1 | 2 | 6 | 2 | 1 | 31 |
| After 6 months | 2 | 2 | - | - | 2 | 7 | 10 | 3 | 5 | 31 |
| Total | 7 | 10 | 2 | 5 | 3 | 9 | 18 | 5 | 6 | 65 |
Others – Non-rejection causes.
Histological diagnosis in category 6, with or without superimposed rejection, corresponding to the timing of biopsies.
| Biopsy timing | CNIT | CNIT + BKVN | BKVN | APN | ATN | AH | TMA | CreGN | CGN | FSGS | Oxalosis | Total |
|---|
| Within 1st week | | | | | 2 | | | | | | | 2 |
| -1st week - 1st month | 3 | 1 | | | 3 | | | | | | 2 | 9 |
| >6 months | 7 | 1 | 2 | 1 | | 1 | 3 | 1 | 1 | 1 | | 18 |
| Total | 10 | 2 | 2 | 1 | 5 | 1 | 3 | 1 | 1 | 1 | 2 | 29 |
ATN- Acute tubular necrosis, AH- Arteriolar hyalinosis, APN- Acute pyelonephritis, BKVN- BK viral nephropathy, CNIT- Calcineurin Inhibitor Toxicity, CGN- Collapsing glomerulopathy, CreGN- Crescentic glomerulonephritis, FSGS- Focal segmental glomerulosclerosis, TMA- Thrombotic microangiopathy.
The most common cause of GD in the 1st week was ATN (2/3, 66.7%) followed by AMR (1/3, 33.3%). After 1st week to 6th month, acute rejection (20/31, 64.5%) was the commonest cause followed by acute CNI Toxicity (4/31, 12.9%), ATN (3/31, 9.7%), de novo renal disease (2/31, 6.4%) (oxalosis) and infection. After 6 months, chronic rejection (19/31, 61.3%) was the commonest cause followed by chronic CNI Toxicity (8/31, 25.8%), infections (4/31, 12.9%), TMA (3/31, 9.7%), de novo glomerulonephritis (3/31, 9.7%) (including Collapsing glomerulopathy, Focal segmental glomerulosclerosis and Crescentic glomerulonephritis) and arteriolar hyalinosis (donor related) (1/31, 3.2%) [Table/Fig-4,5 and 6].
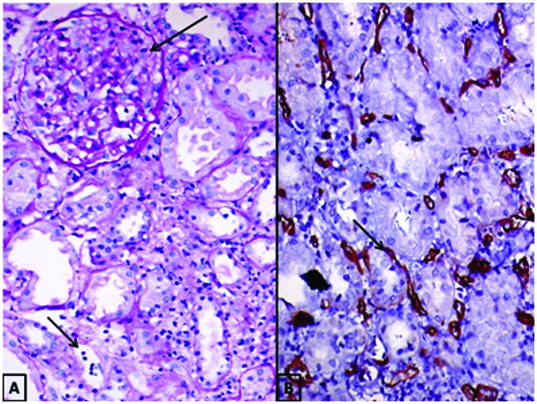
Out of the 35 biopsies of AMR, 18 were acute AMR and 17 were chronic active AMR. C4d deposition along the PTC was present only in 14 (14/35, 40%) biopsies, i.e., 10 of acute AMR (10/18, 55%) and 4 of chronic active AMR (4/17, 23.5%) cases. These comprised of 7 with “diffuse” staining, 6 with “Focal” staining and 1 with “Minimal” staining. The sensitivity of C4d in detecting acute AMR was 55% and chronic AMR was 23.5%. The comparison of C4d positivity with microvascular inflammation {transplant glomerulitis (g>0) and or peritubular capillaritis (ptc>0)} is given in [Table/Fig-7]. [Table/Fig-8] shows a case of AMR with moderate transplant glomerulitis (g2; 25%-75% of glomeruli with inflammation) and moderate peritubular capillaritis (ptc2; >10% of PTCs with 5-10 luminal inflammatory cells) with corresponding C4d immunostaining showing a score of C4d3.
Comparison of C4d positivity with microvascular inflammation.
| C4d Score | Peritubular capillaritis score(total 14) | p-value | Glomerulitis score(total no. 14) | p-value |
|---|
| PTC 0 | PTC 1 | PTC 2 | g 1 | g 2 |
|---|
| C4d 3(n=7) | 0 | 5 (71.4%) | 2(28.6%) | 0.16 | 5 (71.4%) | 2 (28.6%) | 0.29 |
| C4d 2(n=6) | 2 (33.3%) | 3 (50%) | 1 (16.7%) | 0.62 | 5(83.3%) | 1(16.7%) | 0.08 |
| C4d1(n=1) | 0 | 1(100%) | 0 | 0.33 | 1(100%) | 0 | 1.00 |
| Total | 2 | 9 | 3 | | 11 | 3 | |
g1- < 25% of glomeruli with inflammation, g2- 25%-75% of glomeruli with inflammation, ptc 0- < 10% of PTCs with inflammation, ptc 1- > 10% PTCs with <5 luminal inflammatory cells, ptc 2- > 10% of PTCs with 5-10 luminal inflammatory cells.
(A) AMR with moderate transplant glomerulitis (long arrow) and moderate peritubular capillaritis (short arrow) PAS X 200. (B) C4d immunohistochemistry with diffuse peritubular capillary deposits of C4d (Score: C4d3) (arrow) X200.
Even though no PTC C4d deposits (C4d0) were present in the remaining 21 biopsies (i.e. 8 acute AMR and 13 chronic active AMR), these were diagnosed as C4d Negative AMR as they showed characteristic histologic evidence of tissue injury, moderate to severe microvascular inflammation {(g+ptc) ≥2} and serological evidence of donor-specific antibodies (DSA) positivity, thereby, fulfilling the 2013 Banff criteria for AMR [6].
Discussion
Histopathological evaluation is crucial to differentiate diverse causes of GD. The Banff schema provides specific morphological criteria for diagnosis of AMR and TCR. This helps in avoiding over-diagnosis and therefore overtreatment with immunosuppression. It also helps to distinguish “other” inflammatory and “fibrosing” processes that affect the allograft [7,8].
In the present study 38.5% of the biopsies showed histological features involving more than one Banff diagnostic categories which is in synchrony with North Indian study conducted by Philip et al., where 41.4% of the biopsies showed histological features involving more than one group [9].
Philip et al., evaluated 119 biopsies of which majority (47.1%) were in the non-rejection category followed by TCR (31.9%), AMR (28.6%), IFTA (12.6%), borderline changes (7.6%) and normal (4.2%) [9]. Aryal G et al., evaluated the histopathology of 98 graft biopsies of which 24.7% were rejection, 14.3% were due to non-rejection causes, 50.1% were normal, 1% was due to IFTA and 9.2% were non-diagnostic in contrast to our series where majority were rejection (44.6%) followed by non-rejection causes (27.7%), rejection with superimposed non-rejection causes (16.9%) and normal morphology (10.8%) [1]. Such discrepant histological findings could be due to differences in kidney source (cadaver, living related/ un-related), donor and recipient age disparity, race and genetic variability, HLA match, presensitization, type of immunosuppressive protocols, methods of case identification, timing of biopsy, variability of renal lesions and expertise of the Pathologist in recognizing histological differences and distinguishing between Banff diagnostic categories. Further in the latter two studies TCR was the predominant type of rejection compared to AMR (31.9% vs. 28.6% and 8.16 % vs. 6.12% respectively) unlike our series where AMR was predominant over TCR (53.8% vs. 27.8%).
Nickeleit et al., stated that “Acute rejection episodes can be ‘pure’ antibody or ‘pure’ cellular mediated events or represent mixed rejection with varying degrees of humoral and cellular components” [10]. The authors believed that mixed AMR+TCR are commoner than their pure counterparts, as 20%-30% of TCR type I (Banff category 4, tubulointerstitial inflammation), 40%-50% of TCR type II (Banff category 4, transplant endarteritis) and 60% of the grafts with transplant glomerulitis are C4d positive and fall into mixed ABMR+TCMR group [10]. These observations are further substantiated by our study where, of the 40 cases with immunological rejection, 25% were ‘pure’ AMR, 37.5% were mixed AMR + TCR and 8% were ‘pure’ TCR. The authors further emphasise the importance of identifying mixed rejection episodes as they behave differently from ‘pure’ AMR, require intense anti-T cell therapy and are clinically more severe than TCR episodes [10]. This has been our experience also.
Philip et al., observed that non-rejection pathology forms an important cause of renal dysfunction in RT patients [9]. ATN (25.2%) comprised the largest group of non-rejection category in their study followed by CNI toxicity (16%) and infection (10.9%). In our study CNI toxicity was the commonest (41.4%) which is compatible with study conducted by Solez et al., (42.9%) [11]. ATN comprised 17.2% of our non-rejection cases which is in synchrony with Mazzali et al., (19.5%) [9,12]. We observed infection in 17.2% of non-rejection group composed of BKVN (80%) and acute pyelonephritis (20%) whereas, Philip et al., identified BKVN (69.2%), tuberculosis (23.1%) and mucormycosis (7.8%) [9].
Our frequency of de novo glomerulonephritis was 4.6% which is slightly higher than the frequencies of 0.6% to 2.5% as quoted by other studies [9]. FSGS was the commonest de novo disease in these studies.
In the early post-transplant period (0-6 months), the most common cause of GD was Acute rejection, followed by ATN and acute CNI Toxicity. These observations are in synchrony with the findings of Aryal G et al. In the 1st week ATN was the commonest cause followed by AMR.
Literature review reveals that chronic rejection, CNI Toxicity, Infection (BKVN), Recurrent disease, de novo disease (diabetic nephropathy), de novo arteriosclerosis (hypertensive vascular disease), Renal artery stenosis, urinary tract obstruction and IFTA are the causes of late graft dysfunction [3]. In the present study, chronic rejection was the commonest cause of GD in the late post transplant period (> 6 months post-transplantation) followed by chronic CNI Toxicity, infections, TMA and de novo glomerulonephritis. Interestingly, we did not encounter upon any case of diabetic nephropathy.
In current study, a diagnosis of AMR was rendered when the following three features were present: (A) Histologic evidence of acute tissue injury (including one or more of the following: i) microvascular inflammation; ii) Intimal or transmural arteritis; iii) Acute thrombotic microangiopathy, in the absence of any other cause; iv) Acute tubular injury for acute/ active AMR or morphologic evidence of chronic tissue injury (including one or more of the following: i) Transplant glomerulopathy; ii) Severe peritubular capillary basement multilayering; iii) arterial intimal fibrosis; iv) for chronic active AMR. (B) Evidence of current/ recent antibody interaction with vascular endothelium (including one or more of the following: i) C4d staining in peritubular capillaries; ii) At least moderate microvascular inflammation. (C) Serologic evidence of donor specific antibodies.
C4d is a degradation product of the activated complement factor C4 that has a thioester moiety which enables strong covalent bonding with the amino or hydroxyl containing molecules of endothelial cells and basement membrane [13]. Detection of C4d (by IF/IHC) is regarded as an indirect sign/ footprint of an antibody response [14]. Banff 2007 incorporated PTC C4d staining as one of the diagnostic triad for chronic active AMR along with histopathological features of tissue injury and presence of donor-specific antibody (DSA) [15,16]. As C4d linked DSA with histopathology and predicted allograft failure, it became the corner stone of AMR diagnosis in clinical practice [15]. However, recent data have questioned the sensitivity and specificity of C4d staining [6,17]. Many studies have supported the existence of AMR with negative PTC C4d deposition culminating in the revision of AMR criteria by the Banff 2013 conference with inclusion of “C4d-Negative ABMR” [6]. Takeda A et al., found C4d positivity in 46.9% of AMR cases (62.5% positivity in acute AMR and 31.3% in chronic AMR) which is in synchrony with our study (40%) (55.6% in acute AMR and 23.5% in chronic AMR) [16].
The potential cause of C4d negativity include complement independent pathways of endothelial activation, C4d deposition in low amounts beyond the detection limits of IF/IHC, technical factors inherent in the methodology, treatment effects, and, fluctuation of C4d status in the first year post transplantation[3,16,17]. Thus C4d alone is not sensitive enough to diagnose AMR. Recent focus is on molecular markers like ENDATs (endothelial cell activation-associated transcripts) as indicator of active endothelial injury/ ABMR [6,16].
Several studies have shown statistically significant correlation between PTC C4d deposition and microvascular inflammation [18,19]. In our series, although there was an increase in the proportion of moderate microvascular inflammation (ptc2 and g2) in diffuse C4d positive cases (C4d3) compared to focal C4d positivity (C4d2) (28.6% in C4d3 and 16.7% in C4d2), this difference was not statistically significant.
Conclusion
AMR and CNI Toxicity account for majority of graft dysfunction. The most common cause of early GD was acute rejection followed by ATN and acute CNI toxicity. The commonest cause of late GD was chronic rejection followed by chronic CNI Toxicity and infections. C4d is not as sensitive a marker of AMR, as was initially thought. Higher proportion of moderate microvascular inflammation was found in diffuse C4d positive cases as compared to focal C4d positive cases.
AMR- Antibody mediated rejection, TCR- T-cell-mediated rejection.
AMR- Antibody mediated rejection, ATN- Acute tubular injury, BKVN- BK viral nephropathy, CNI- Calcineurin Inhibitor, FSGS- Focal segmental glomerulosclerosis, TCR- T-cell-mediated rejection, TMA- Thrombotic microangiopathy.
Others – Non-rejection causes.
ATN- Acute tubular necrosis, AH- Arteriolar hyalinosis, APN- Acute pyelonephritis, BKVN- BK viral nephropathy, CNIT- Calcineurin Inhibitor Toxicity, CGN- Collapsing glomerulopathy, CreGN- Crescentic glomerulonephritis, FSGS- Focal segmental glomerulosclerosis, TMA- Thrombotic microangiopathy.
g1- < 25% of glomeruli with inflammation, g2- 25%-75% of glomeruli with inflammation, ptc 0- < 10% of PTCs with inflammation, ptc 1- > 10% PTCs with <5 luminal inflammatory cells, ptc 2- > 10% of PTCs with 5-10 luminal inflammatory cells.