Thyroid cancer is the most common endocrine malignancy, and more than 95% of thyroid carcinomas originate from follicular epithelial cells [1]. Pathologists can differentiate between benign and malignant lesions in most of thyroid tumours using only the histopathologic criteria, thereby, assuring an accurate diagnosis and classification of thyroid neoplasm.
However, in most cases, the pathologists are confronted with thyroid lesions in which the distinction between benign and malignant can be quite elusive. The decision favouring one or another has clinical consequences and implies different modalities of treatment. While evading excessive treatment and psychological discomfort to the patient is a necessity, yet proper effective management of patients with potentially aggressive diseases at early curable stages should be assured [2]. Accordingly, ancillary techniques, immunohistochemistry and molecular profiling can enhance the standard morphologic assessment in such challenging cases [3].
Galectin-3 is a protein that binds to-galactosidase residues on cell surface glycoproteins and has also been identified in the cytoplasmic and nuclear compartment [4]. In the thyroid, several reports have shown that galectin-3 is over expressed in malignant tumours [5,6]. Galectin-3 detection by immunohistochemistry has been used to improve diagnostic accuracy in preoperative evaluation of thyroid nodules [7,8]. Galectin-3 may be a helpful tool for classical PTC, but it cannot be considered a diagnostic marker of malignancy [9]. Glypican-3, a member of the glypican family of heparan-sulfate proteoglycans (HSPGs), is bound to the plasma membrane through a glycosyl phosphatidylinositol (GPI) anchor [10]. Further studies underlined the important role of glypican-3 in regulation of cell proliferation and apoptosis during normal development [11]. Glypican-3 is down-regulated in several malignant tumours, such as ovarian carcinoma, cholangiocarcinoma, mesothelioma, and breast cancer, due to hypermethylation of the glypican-3 promoter [12,13]. Glypican-3, is not thoroughly studied in the thyroid, few studies like Yamanaka reported its scarce expression in the normal thyroid gland, but it is dramatically enhanced in certain types of thyroid cancers as in follicular carcinoma and in papillary carcinoma [14].
In the current study, we aimed to evaluate Glypican-3 and Galectin-3 in thyroid carcinomas and adenomas. The expression of both markers was first compared between all thyroid carcinoma and adenoma generally, and then compared between follicular-patterned thyroid carcinomas and thyroid adenoma.
Materials and Methods
In this study, we investigated the differential expression of both Glypican-3 and Galectin-3 in thyroid neoplasm. This study was conducted on archival blocks diagnosed from pathology department between 2010 and 2012 included 17 cases of follicular adenoma, 16 cases of classic Papillary Thyroid Carcinoma (PTC), 6 cases of Follicular Variant Of Papillary Thyroid Carcinoma (FVPTC), 3 cases of follicular carcinoma, 5 cases of medullary carcinoma and 1 case of Hürthle cell carcinoma. The nearby non neoplastic (normal) thyroid follicles present in both adenoma and carcinoma cases were also evaluated.
Histopathological evaluation
The sections stained with H&E were examined by two pathologists under the light microscope for confirmation of the diagnosis of follicular adenoma and thyroid carcinoma.
Immunohistochemical staining of glypican-3 and galectin-3
Immunohistochemical staining of both glypican-3 and galectin-3 was performed on cross-sections derived from selected paraffin blocks. Blocks that were suitable for the study were selected by two pathologists. Formalin-fixed paraffin-embedded thyroid tissues were cut at 4μm thickness, mounted onto poly-L-lysine precoated positively charged glass slides. All slides were de-paraffinized using xylene and then rehydrated in decreasing concentrations of ethanol. Antigen retrieval using microwave heating (20 minutes; 10 mmol/citrate buffer, pH 6.0) after inhibition of endogenous peroxidase activity (hydrogen peroxidase for 15 min) was used. The primary antibodies anti-galectin-3 monoclonal antibody (Lab Vision Corporation 46360 Fremont Blvd. Fremont, CA 94538-6406, USA, 0.5ml concentrated,1:50 dilution) and with anti glypican-3 antibody (BIOCARE, MEDICAL, LLC, USA, 6 ml prediluted) were applied to the slides, incubated overnight at room temperature in a humidity chamber. The slides were then covered in mouse serum blocking reagent for 15 minutes, followed by avidin and biotin blocking reagents for 15 minutes each. They were subsequently counterstained with haematoxylin and mounted for microscopic evaluation. Hepatocellular carcinoma was used as a positive control for glypican-3 and small bowel for galectin-3 as provided by the datasheet of both primary antibodies.
Interpretation of immunohistochemical results
The slides were screened and observed by two pathologist. The morphology and cytological appearances were recorded. The evaluation was done by the semiquantitative scoring method as referenced by Ho et al., [15]. Scoring was done based on the intensity of the staining characteristics on a scale of 1 to 3: a score of 1 for focal/weak staining; a score of 2 for moderate staining; and a score of 3 for strongly positive staining reaction [15]. The average scoring among the two pathologists was calculated. All fields in a given specimen were individually scored, the percentage of fields determined, and the scores added to yield an average staining intensity score for the entire specimen. Scoring was done carefully on cells at the central part (C) of the lesion and cells at the periphery (P), and adjacent to the neighboring normal thyroid tissue [16].
Statistical Analysis
Descriptive analysis of the variables and statistical significances were calculated by non-parametric chi-square test using the Statistical Package for the Social Sciences version 12.0 (SPSS). Values were expressed in number, percentage. Fischer’s-exacts test was used to evaluate exact probabilities in a contingency table (usually 2x2) when the expected frequencies were small and X2 (chi square) when the expected frequencies were large. Differences were considered statistically significant with p< 0.05 and highly significant when p-value <0.01. Sensitivity: probability that a test result will be positive when the disease is present (true positive rate). Specificity: probability that a test result will be negative when the disease is not present (true negative rate).Positive predictive value: probability that the disease is present when the test is positive. Negative predictive value: probability that the disease is not present when the test is negative. Diagnostic accuracy: true positive cases + true negative cases/ true positive + true negative + false positive +false negative [17].
Results
[Table/Fig-1] shows the cases included in this study, with a total number of 17 cases of follicular adenoma and 31 case of thyroid carcinoma. The carcinoma cases included 16 (52%) cases of classic papillary thyroid carcinoma, 6 (19%) cases of follicular variant of papillary thyroid carcinoma, 5 (16%) cases of medullary carcinoma 3 (10%) cases of follicular carcinoma and 1 (3%) case of Hürthle cell carcinoma, which are shown microscopically in [Table/Fig-2].
Cases included in the study.
| Studied groups | Number of cases |
|---|
| Follicular adenoma | 17 cases |
| Thyroid carcinoma | 31 cases |
| • Classic papillary | 16 cases |
| • FVPT | 6 cases |
| • Follicular carcinoma | 3 cases |
| • Medullary carcinoma | 5 cases |
| • Hürthle cell carcinoma | 1 case |
FVPT: Follicular Variant of Papillary Thyroid carcinoma
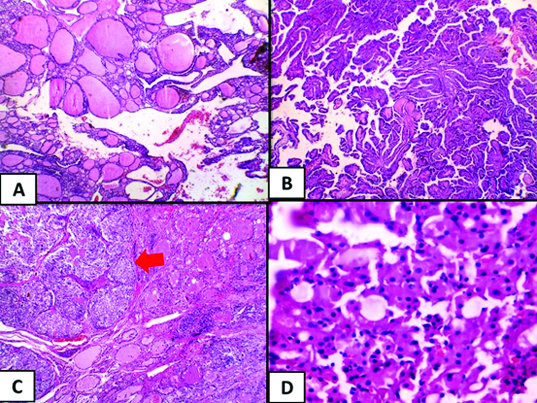
A: Follicular adenoma (H&E, 10x) B: Papillary thyroid carcinoma, classic type. (H&E, 4x) C: Medullary carcinoma (red arrow) (H&E, 4x) D: Hürthle cell carcinoma (H&E, 20x).
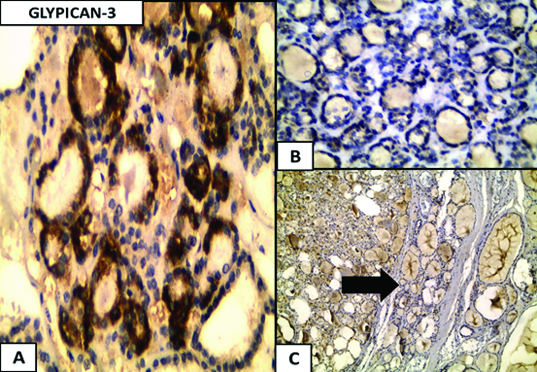
A positive staining reaction was noted as cytoplasmic brown granular stain in the thyroid epithelial cells regarding both the galectin-3 and glypican-3 antibodies. It varied from diffused extensive deposition to fine granularity. Macrophages and red blood cells within vascular spaces that also showed cytoplasmic granular staining were excluded from scoring. Galectin-3 and glypican-3 were expressed in 97% and 81% of the carcinoma cases, respectively, while 60% and 76% of the adenoma cases negatively expressed both galectin-3 (p=0.0006) and glypican-3 (p=0.0002) respectively [Table/Fig-3]. The adjacent normal in carcinoma and adenoma cases showed complete absence of positive stain reaction regarding both galectin-3 and glypican-3 antibodies, while areas of papillary hyperplasia showed strongly diffuse positive staining for both galectin-3 and glypican-3 [Table/Fig-4].
Galectin-3 and Glypican-3 expression in the studied cases.
| AdenomaNo. % | CarcinomaNo. % | Test of significance | p-value |
|---|
| Galectin-3 Expression | p=0.0006** |
| Positive | 5 | 30% | 30 | 97% | FE |
| Negative | 12 | 60% | 1 | 3% |
| Glypican-3 expression | p=0.0002** |
| Positive | 4 | 24% | 25 | 81% | FE |
| Negative | 13 | 76% | 6 | 19% |
a) Glypican-3 expression in follicular adenoma, cytoplasmic expression is noticed in the follicular adenoma, (Immunoperoxidase 40x); b) Negative expression in follicular adenoma (Immunoperoxidase 40x); c) Negative expression in the nearby normal thyroid tissue (black arrow) (c) (Immunoper oxidase 4x).
[Table/Fig-5] shows the expression of both galectin-3 and glypican-3 regarding the type of studied thyroid carcinoma cases, for all the cases of classic papillary thyroid carcinoma (16/16), follicular variant of papillary thyroid carcinoma (6/6), medullary carcinoma (5/5), Hürthle cell carcinoma cell carcinoma (1/1) and 67% (2/3) of follicular carcinoma expressed galectin-3 [Table/Fig-6]. While glypican-3 expression was recorded in all cases of follicular variant of papillary thyroid carcinoma (6/6), Hürthle cell carcinoma cell carcinoma (1/1), 80% (4/5) of medullary carcinoma, 75% (12/16) of papillary thyroid carcinoma and 67% (2/3) of follicular carcinoma [Table/Fig-7].
expression of galectin-3 and glypican-3 according to type of thyroid carcinoma.
| carcinoma |
|---|
| PTCNO (%) | FVPTNO (%) | FCANO (%) | MEDULLARYNO (%) | HÜRTHLE CELL CARCINOMANO (%) |
|---|
| Galectin-3 Expression |
| Negative | 0 | 0 | 1 (33%) | 0 | 0 |
| Positive(20) | 16 (100%) | 6(100%) | 2 (67%) | 5 (100%) | 1 (100%) |
| Total | 16 (100%) | 6(100%) | 3 (100%) | 5 (100%) | 1 (100%) |
| Glypican-3 Expression |
| Negative | 4(25%) | 0 | 1 (33%) | 1 (20%) | 0 |
| Positive (19) | 12 (75%) | 6(100%) | 2 (67%) | 4 (80%) | 1 (100%) |
| Total | 16 (100%) | 6(100%) | 3 (100%) | 5 (100%) | 1 (100%) |
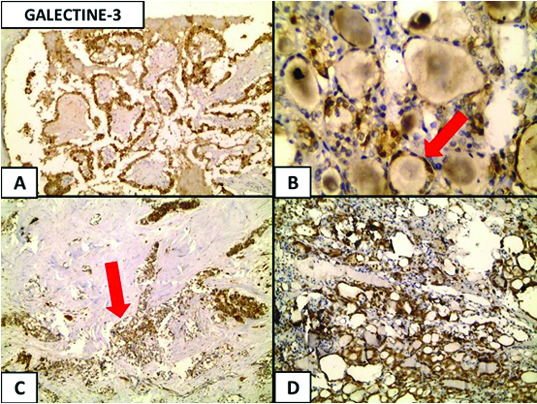
(a) Galectine-3 expression in thyroid carcinoma, a cytoplasmic expression is noticed in the papillary thyroid carcinoma (Immunoperoxidase 20x); (b) Follicular carcinoma (red arrow) (Immunoperoxidase 40x); (c) medullary carcinoma (red arrow); (Immunoperoxidase 4x); and (d) in Hürthle cell carcinoma (Immunoperoxidase 4x).
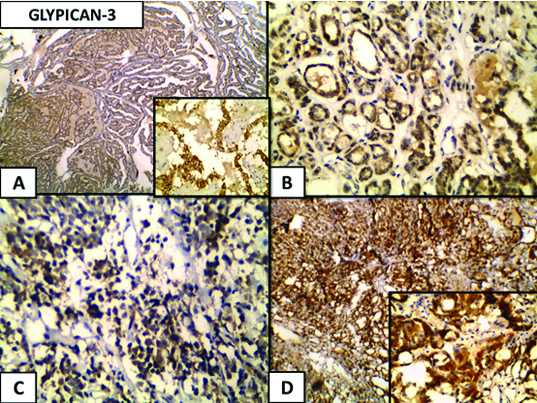
(a) Glypican-3 expression in thyroid carcinoma, a cytoplasmic expression is noticed in the papillary thyroid carcinoma (Immunoperoxidase 4xand 40x inset); (b) Follicular carcinoma (Immunoperoxidase 40x); (C) Medullary carcinoma (Immunoperoxidase 40x); and (d) Hürthle cell carcinoma (Immunoperoxidase 4x and 40x inset).
The follicular-patterned thyroid malignancy included any thyroid malignancy arranged in follicular pattern, which in the current study involved the follicular carcinoma (3 cases), the follicular variant of papillary thyroid carcinoma (6 cases) and Hürthle cell carcinoma cell carcinoma (1 case). The expression of both galectin-3 and glypican-3 in follicular-patterned thyroid malignancy (10 cases) vs. follicular adenoma revealed significant difference P= 0.0044 and P= 0.0013, respectively. The expression in follicular-patterned thyroid malignancy vs. follicular adenoma was 90% vs.30% for galectin-3 and 90% vs. 24% for glypican-3 expression [Table/Fig-8].
Expression of galectin-3 and glypican-3in follicular-patterned thyroid carcinoma vs. follicular adenoma.
| Follicular adenomaNo. (17) %(100%) | Follicular-patterned carcinomaNo. (10) % (100%) | Test of significance | p-value |
|---|
| Galectin-3 Expression | p=0.0044** |
| Positive | 5 | 30% | 9 | 90% | FE |
| Negative | 12 | 60% | 1 | 10% |
| Glypican-3 expression | p=0.0013** |
| Positive | 4 | 24% | 9 | 90% | FE |
| Negative | 13 | 76% | 1 | 10% |
P: peripheral C: central
A strongly positive staining intensity was noted, preferably in the peripheral cells adjacent to the neighboring normal thyroid tissue (P) and a weaker staining intensity in the cells at the central portion (C) was noted in both galectin-3 (23%) and glypican-3 (16%) stained thyroid carcinoma. while the vast majority of the galectin-3 (77%) and glypican-3 (84%) positively expressed carcinoma revealed an equal distribution between the periphery and the center of the tumour [Table/Fig-9].
Comparison between the distribution of galectin-3 and glypican-3 in the carcinoma and adenoma groups.
| Adenoma | Carcinoma | Test of significance | p-value |
|---|
| Galectin-3 Expression distribution | p= 0.13 |
| P=c | 2 | 40 | 23 | 77% | FE |
| P>c | 3 | 60 | 7 | 23% |
| 5 | 100% | 30 | 100% | | |
| Glypican-3 expression Distribution | p= 0.55 |
| P=c | 3 | 75 | 21 | 84% | FE |
| P>c | 1 | 25 | 4 | 16% |
| 4 | 100% | 25 | 100% | | |
Regarding the intensity of the stain of expression, 70% of the carcinoma cases exhibited strong galectin-3 expressions while 40% of the adenoma cases revealed strong galectin-3 expressions. The difference in the intensity of expression of galectin-3 in the studied groups failed to achieve a statistical significance [Table/Fig-10].
Comparison between the intensity of galectin-3 and glypican-3 expression in the carcinoma and adenoma groups.
| Adenoma | Carcinoma | Test of significance | p-value |
|---|
| Galectin-3 intensity | p= 0.39 |
| Weak | 2 | 40 | 5 | 17% | χ2= 1.89 |
| Moderate | 1 | 20 | 4 | 13% |
| Strong | 2 | 40 | 21 | 70% |
| 5 | 100% | 30 | 100% |
| Glypican-3 expression Distribution | p= 0.36 |
| Weak | 0 | 0 | 4 | 16% | χ2= 2.04 |
| Moderate | 1 | 25 | 12 | 48% |
| Strong | 3 | 75 | 9 | 36% |
| 4 | 100% | 25 | 100% |
The sensitivity, specificity, positive predictive value, negative predictive value and diagnostic accuracy of both galectin-3 and glypican-3 in discrimination between adenoma and carcinoma, and in between follicular adenoma and follicular-patterned thyroid carcinoma cases, are shown in [Table/Fig-11]. In discrimination between adenoma and all thyroid carcinoma cases, galectin-3 was more sensitive (96.8%) while glypican-3 was more specific (76.5%). As for the discrimination between adenoma and follicular-patterned thyroid carcinoma cases, glypican-3 was more specific (76.5%), while both markers were of the same sensitivity.
Sensitivity, Specificity, Positive predictive value, Negative predictive value and Diagnostic accuracy of both Galectin-3 and Glypican-3 in Discrimination between adenoma and all thyroid carcinoma cases and between adenoma and follicular-patterned thyroid carcinoma cases.
| Discrimination between adenoma and all thyroid carcinoma cases |
|---|
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | Diagnostic accuracy |
|---|
| Galectin-3 | 96.8% | 70.6% | 85.7% | 92.3% | 87% |
| Glypican-3 | 81% | 76.5% | 86% | 68% | 79% |
| Discrimination between adenoma and follicular-patterned thyroid carcinoma cases |
|---|
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | Diagnostic accuracy |
|---|
| Galectin-3 | 90% | 71% | 64% | 92% | 78% |
| Glypican-3 | 90% | 76.5% | 69% | 93% | 82% |
Discussion
Galectin-3 initially was found in some studies to be a good marker of malignancy, distinguishing PTC and follicular carcinomas from benign conditions [5,18–20]. However, several additional studies have shown galectin-3 expression in benign conditions and follicular adenomas, in addition to staining inflammatory cells and reactive normal epithelium, dampening the usefulness of this marker in discriminating benign from malignant [21–24].
As for the glypican-3, its role in hepatocellular carcinoma has been meticulously studied. Glypican-3 was found to be frequently expressed in HCCs and, therefore, possibly function as a diagnostic marker [25–28]. While in the thyroid, although its role and expression in thyroid neoplasm is not thoroughly studied, yet according to Yamanaka and colleagues in 2007, glypican-3 is overexpressed in cancer lesions and scarce in the normal and adenomatous thyroid tissue [14].
Galectin-3 was found to be frequently expressed in thyroid carcinomas represented as the most well-studied molecular candidate for thyroid cancer diagnosis [5,6,29,30] and [9]. In our series, galectin-3 was expressed in all cases of classic papillary thyroid carcinoma, follicular variant of papillary thyroid carcinoma, medullary carcinoma, Hürthle cell carcinoma and 67% of follicular carcinoma while it was completely absent in the nearby non neoplastic thyroid tissue. Our results are in the same range of percent with previous studies as Fernandez et al., Kovacs et al., Fischer and Asa, Prasad et al., Herrmann et al., and Bartolazzi et al., [5,6,9,23,30,31].
In our study glypican-3 was absent in the nearby non neoplastic thyroid tissue and was recorded in all cases of follicular variant of papillary thyroid carcinoma, Hürthle cell carcinoma, 80% of medullary carcinoma, 75% of papillary thyroid carcinoma and 67% of follicular carcinoma. Yamanaka and colleagues supported our results, regarding the scarce expression in normal thyroid tissue and in papillary carcinoma (70%, 48/69 cases) [14], while a discrepancy was noticed regarding the follicular carcinoma (100% in yamanaka and colleagues vs. 67% in our series). This discrepancy could be attributed to the small sample size of follicular carcinoma in our series.
In our study, galectin-3 was sensitive by 96.8%, specific by 70.6%, and had a diagnostic accuracy of 87.5% in discrimination between thyroid carcinoma and adenoma. Bartolazzi and colleagues underwent a study in 2001 done on 226 specimens of thyroid nodules (188 benign lesions and 34 carcinomas) which reported sensitivity, specificity, positive predictive value, and diagnostic accuracy of galectin-3 immunodetection were 100%, 98%, 92%, and 99%, respectively [31]. In another study of 125 thyroid aspirates (50 follicular adenomas, 33 follicular carcinomas, and 42 papillary carcinomas) the sensitivity, specificity, positive predictive value, and diagnostic accuracy of galectin-3 as a single marker in discriminating benign from malignant lesions were 92%, 94%, 95.8%, and 92.8%, respectively [32].
The small sample size in our study could be the reason for the lower sensitivity, specificity and diagnostic accuracy of galectin-3. However, a study done on a small sample size of thyroid neoplasm (44 follicular adenomas and 35 follicular carcinomas) by Maruta and colleagues in 2004 stated a lower specificity (52%) than our results for galectin-3 [8].
Discriminating follicular-patterned malignancy (as follicular carcinoma, follicular variant of PTC and Hürthle cell carcinoma cell carcinoma) from follicular adenoma may pose a challenge, for the diagnostic criteria may not be fulfilled by histopathologic criteria alone, and an immunohistochemical intervention may be required to confirm diagnosis. In our study, glypican-3 was more specific and more accurate diagnostically in comparison to galectin-3 in discrimination between follicular-patterned thyroid carcinoma and follicular adenoma. However both were of the same sensitivity (90%).
Scognamiglio and colleagues compared HBME1, CK19, galectine-3 and CITED1 in a study done in 2006. They reported that HBME1 was the most specific, and galectine-3 had the lowest specificity among the 4 markers. In contrast, CK19 was the most sensitive for the diagnosis of PTC (papillary thyroid carcinoma) vs FA (follicular adenoma) and PTCC (papillary thyroid carcinoma classic form) vs FA. However, when comparing PTCFV (papillary thyroid carcinoma follicular variant) vs FA, CK19 and GAL3 showed equal sensitivity (90%), higher than CITED1 or HBME1 [33].
Galectin-3 is more sensitive than glypican-3 in discriminating between thyroid carcinoma and adenoma, however glypican-3 was more specific and more accurate diagnostically than galectin-3. No previous studies encountered a comparison between galectin-3 and glypican-3 regarding its sensitivity, specificity and diagnostic accuracy in discrimination between thyroid carcinoma and adenoma.
Limitation
Infrequency of the follicular carcinoma cases contributed to the small sample size of the current study.
Absence of sources of funding to conduct this study or prepare this manuscript, caused limitation in investigating the biological an molecular mechanisms in thyroid maliganancy.
Conclusion
Glypican-3 can be used in association with galectin-3 as a useful diagnostic tool for differentiation between follicular-patterned thyroid malignancy and follicular adenoma. A study on a larger sample size of thyroid neoplasm comparing between both galectin-3 and glypican-3, investigating their biological and molecular mechanisms in thyroid malignancy and specifically in follicular-patterned malignancy is highly recommended.
FVPT: Follicular Variant of Papillary Thyroid carcinoma
P: peripheral C: central