Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) are the most Severe Cutaneous Adverse Reactions (SCARs) which mainly caused by exposure to drugs and having significant morbidity and mortality. TEN represents an immunologic reaction to a foreign antigen and is most often caused by drugs. Nevirapine (NVP), a non-nucleoside reverse transcriptase inhibitor (NNRTI) is an important component of Highly Active Antiretroviral Therapy (HAART). It is sometimes associated with life-threatening adverse reactions. Here, we report the fatal case of 72-year-old male who developed TEN secondary to intake of nevirapine. This fatal case report will increase awareness among treating physicians for careful monitoring of patients on NNRTI-based antiretroviral therapy and better counseling of the patient on NVP regimen for early identification and reporting of SCARs so that fatalities due to adverse drug reactions can be prevented with timely intervention.
Case Report
A case of 72-year-old HIV sero-positive man who was admitted in the emergency with sudden development of diffuse, erythematous skin rash all over the body and difficulty in intake of food since four days following three weeks treatment of a triple combination: Zidovudine (300 mg) + lamivudine (150 mg) and nevirapine (200 mg). NVP was started as 200 mg tablet once daily for 2 weeks and then was increased to twice a day over next 2 weeks. He initially developed itching with maculopapular rash over face, upper portion of chest and trunk. Eight days later, he presented to us with a severe maculopapular rash all over the body with targetoid lesions over the limbs [Table/Fig-1]. Physical examination on admission revealed erythematosus and maculopapular skin rash all over the body including involvement of eye and lips [Table/Fig-2] with body temperature of 38oC.
Haemorrhagic crusting of the lips and multiple erythematous papules, plaques were present all over the body
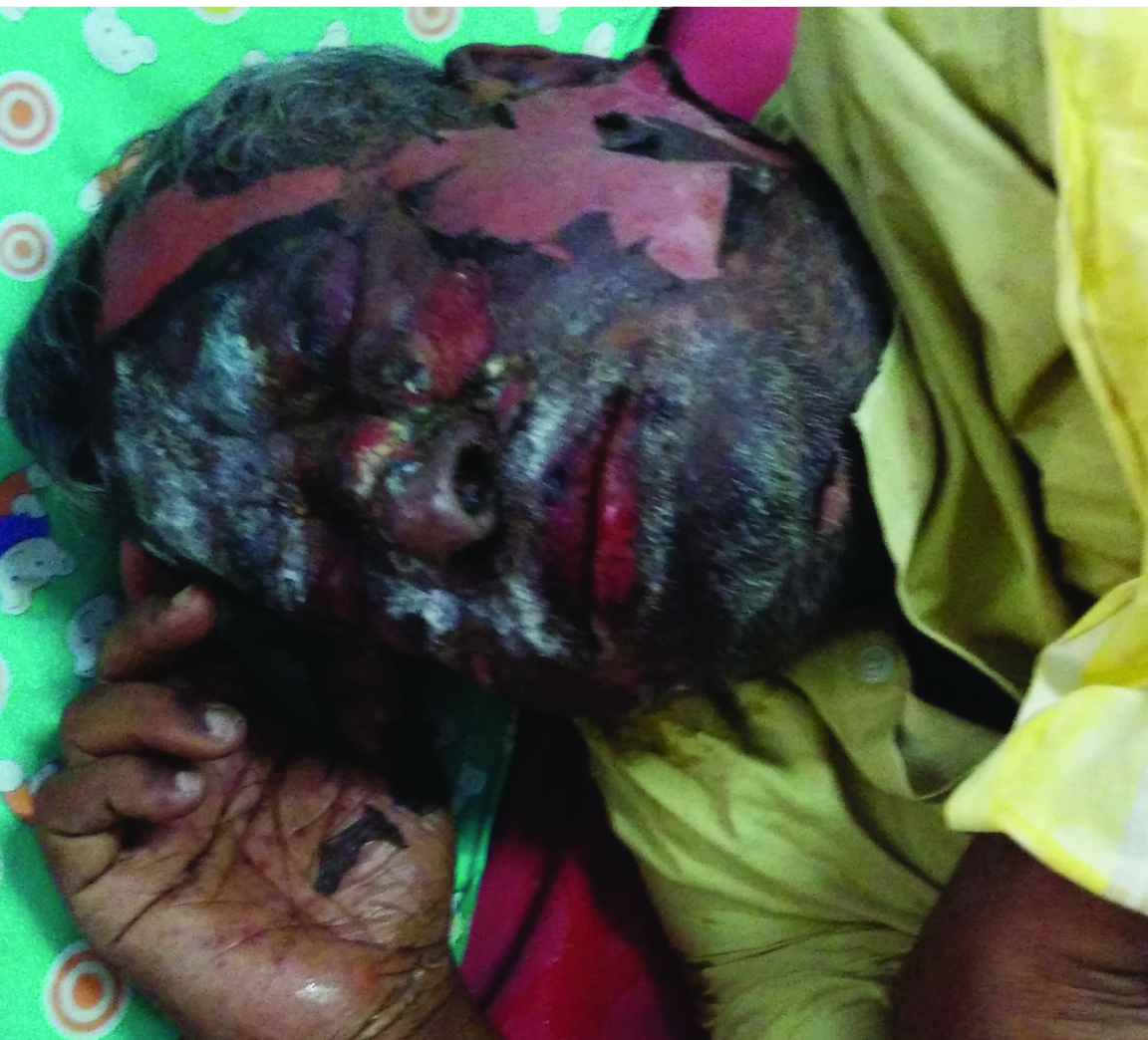
Targetoid lesions over the face, trunk and abdomen as initial manifestation
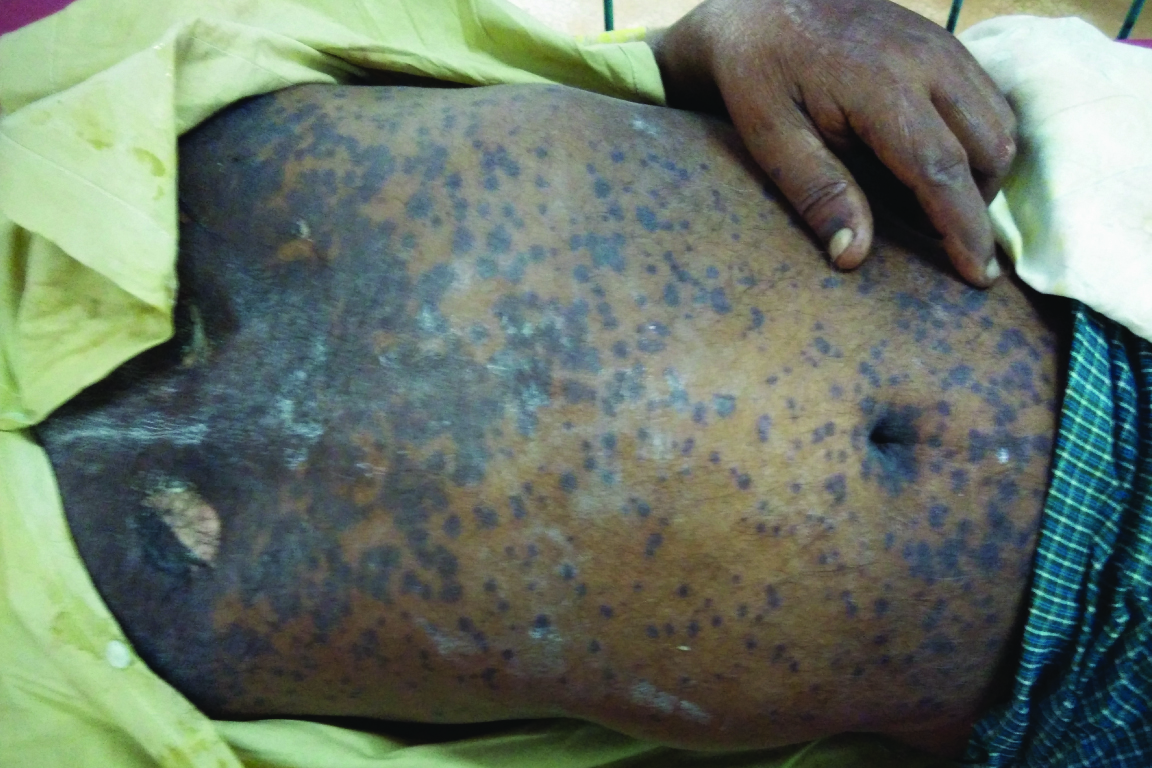
The entire skin covering the face and anterior parts of trunk was denuded and peeled off with minor manipulation and appeared blackish in colour [Table/Fig-3]. Multiple oral ulcers were seen. Haemorrhagic crusting of the lips was also noted [Table/Fig-3]. Ophthalmic examination revealed conjunctivitis. There was also involvement of skin over genital regions. There was no previous history of drug allergy with same drug or others.
Body surface skin over face and trunk denuded and peeled off with minor manipulation (positive Nikolsky sign) and appeared blackish in colour (TEN)
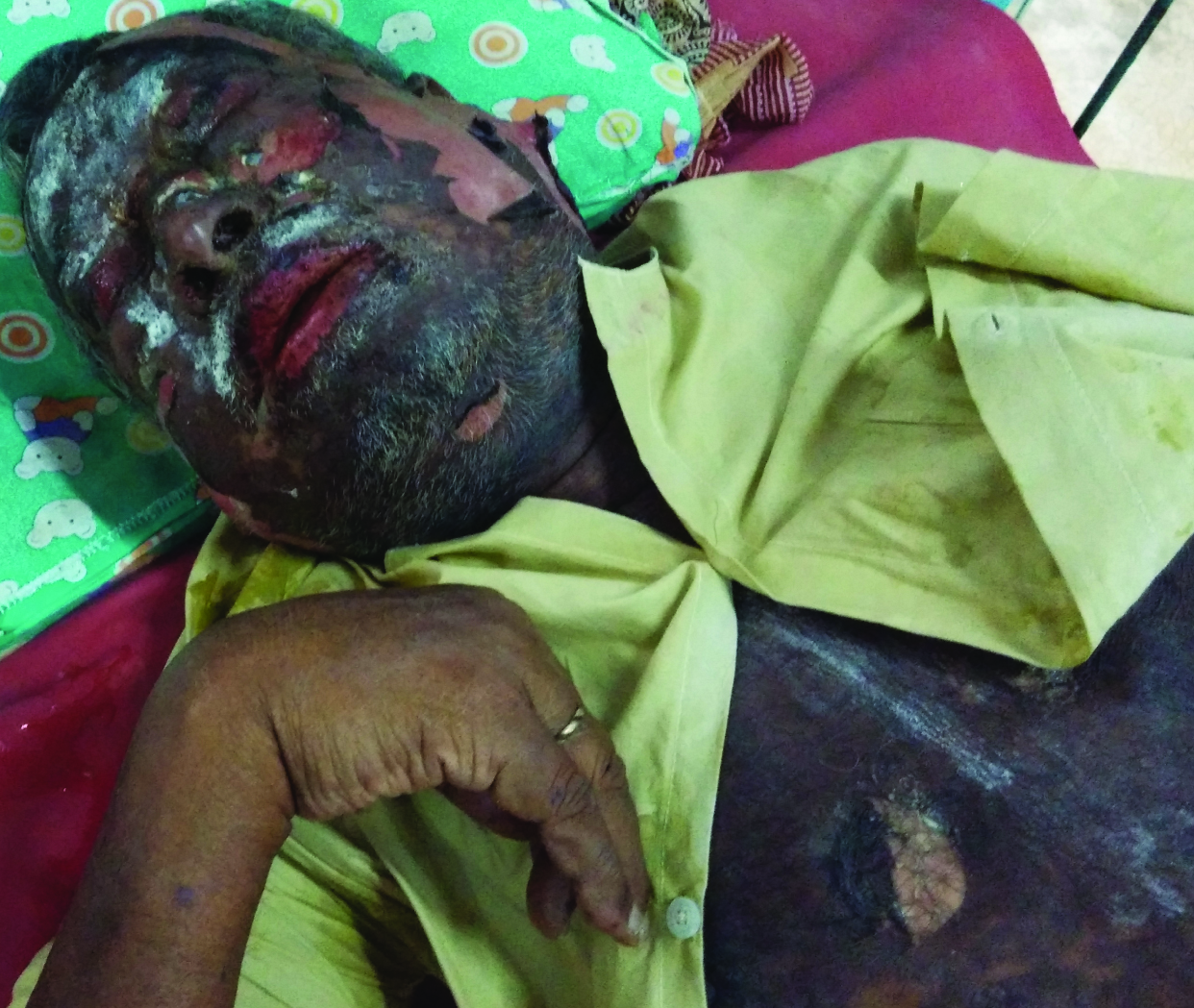
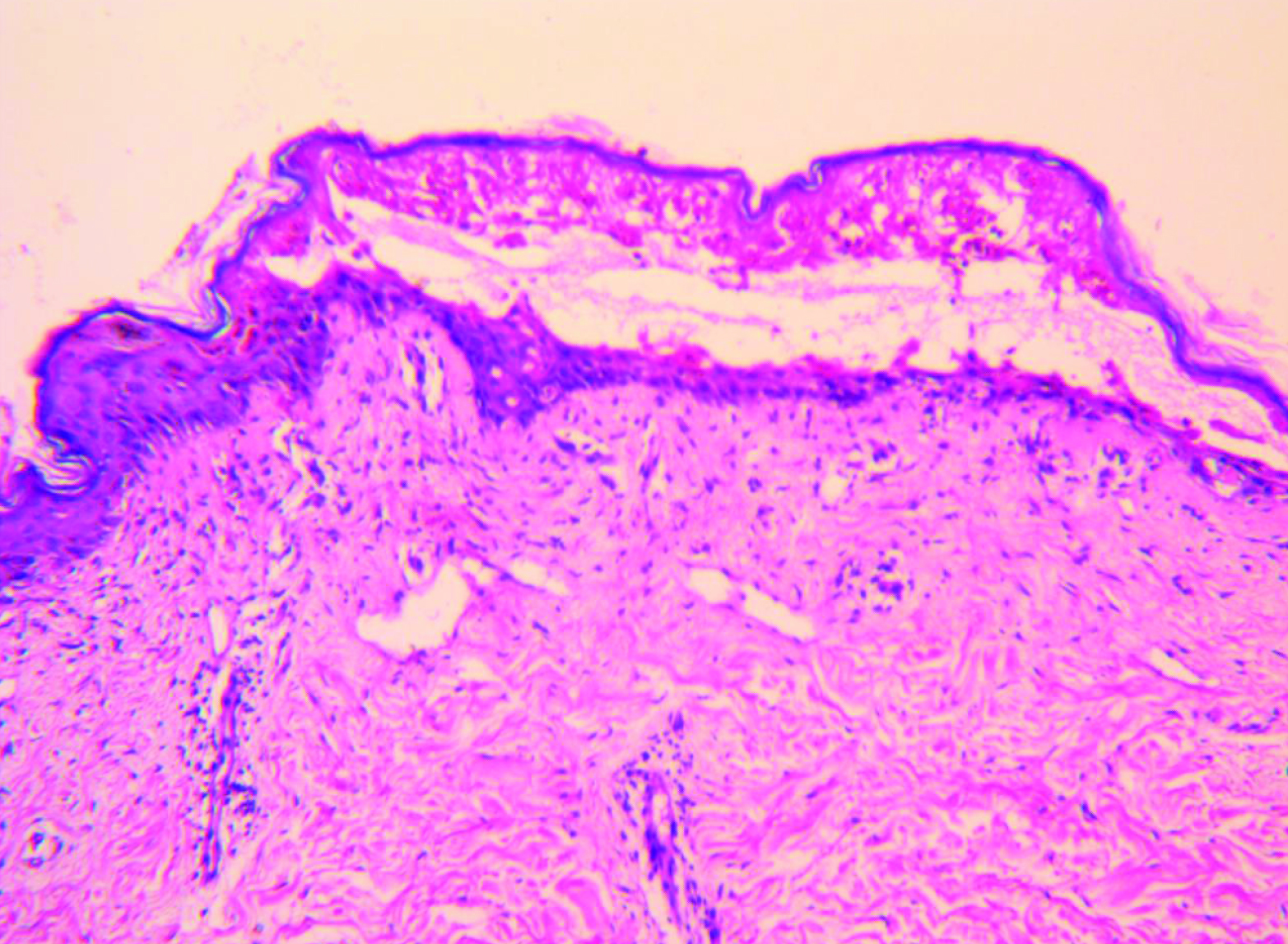
The body surface area involvement of the patient at the time of presentation was approximately 35%. Over the next four days, he developed severe bullous lesions and extensive exfoliation involving more than 60 percent of the body surface, including the palms and soles. A diagnosis of nevirapine-induced TEN was made on the basis of temporal relationship, positive drug history, associated clinical symptoms and signs, positive Nikolsky’s sign and full-thickness epidermal necrolysis on histopathology report [Table/Fig-4]. Serum transaminases (ALT and AST) and other haematological investigations were deranged. Their values were aspartate aminotransferase (1235 U/L) and alanine aminotransferase (790 U/L). There was hyponatraemia (129mEq/L) and hypokalaemia (3.2mEq/L). Blood culture was negative. Serological tests for HSV, HBV and HCV were negative. Other relevant laboratory tests done in hospital (serum urea, glucose and bicarbonate) along with clinical factors gave a SCORTEN of 5 [Table/Fig-5] [1] with a predictive mortality of 90%.
Histopathology of NVP induced toxic epidermal necrolysis
Severity-of-illness assessed by using the SCORTEN criteria
| Prognostic factors | Points | SCORTEN (sum of individual scores) | Predicted mortality (%) | Score in present case |
|---|
| Age > 40 | Yes = 1, No = 0 | 0-1 | 3.2% | 1 |
| Heart rate >120/min | Yes = 1, No = 0 | 2 | 12.1% | 1 |
| Cancer or haematologic malignancy | Yes = 1, No = 0 | 3 | 35.8% | 0 |
| >10% body surface area | Yes = 1, No = 0 | 4 | 58.3% | 1 |
| Serum urea >10mm/L | Yes = 1, No = 0 | >5 | 90% | 1 |
| Serum bicarbonate<20mm/L | Yes = 1, No = 0 | | | 1 |
| Serum glucose >14mm/L(1 mmol/L of Glucose = 18.02 mg/Dl) | Yes = 1, No = 0 | | | 0Total score= 5 |
On hospitalization ART was stopped and patient was put on intravenous immunosuppressant (cyclosporin/CsA), intravenous antibiotic, intravenous paracetamol (as when required, topical antiseptic, anti-histamine, topical lubricants, fluid therapy and parenteral nutrition. The patient was started on immunosuppressant ciclosporin (CsA) at a dosage of 5 mg/kg daily given in 2 divided doses. Wounds were treated conservatively, without skin debridement. Treatment of skin lesions by the topical application of mupirocin, 0.9% NaCl and 0.5% AgNO3 three times a day was done. Despite meticulous supportive care and withdrawal of all suspected drugs, patient’s condition worsened and he died after 7 days of hospital admission.
The causality assessment as per the Naranjo algorithm [2] and WHO- UMC criteria [3] revealed the ADR to be “Probable” (Naranjo score 8). Assessment of causality by using the algorithm of drug causality for epidermal necrolysis (ALDEN) [Table/Fig-6] [4] was also used.
Assessment of causality by using the algorithm of drug causality for epidermal necrolysis (ALDEN).
| Criterion | Values Rules to apply | Score in the present case |
|---|
| Delay from initial drug componentintake to onset of reaction (index day) | Suggestive +3 From 5 to 28 daysCompatible +2 From 29 to 56 daysLikely +1 From 1 to 4 daysUnlikely −1 >56 DaysExcluded −3 Drug started on or after the index dayIn case of previous reaction to the same drug, only changes for:Suggestive: +3: from 1 to 4 daysLikely: +1: from 5 to 56 days | Suggestive +3 |
| Drug present in the body onindex day | Definite 0Doubtful −1Excluded −3 | Definite: 0 |
| Prechallenge/rechallenge | Positive specific for disease and drug: 4Positive specific for disease or drug: 2Positive unspecific: 1Not done/unknown: 0Negative −2 | Not done: 0 |
| Dechallenge | Neutral 0 Drug stopped (or unknown)Negative −2 Drug continued without harm | Neutral: 0 |
| Type of drug (notoriety) | Strongly associated 3 Drug of the “high-risk” list according to previous case–control studiesAssociated 2 Drug with definite but lower risk according to previous case–control studiesSuspected 1 Several previous reports, ambiguous epidemiology results (drug “under surveillance”)Unknown 0 All other drugs including newly released onesNot suspected −1 No evidence of association from previous epidemiology study with sufficient number of exposed controls | Associated: 3 |
| Other cause | Possible −1 Rank all drugs from highest to lowest intermediate scoreIf at least one has an intermediate score >3, subtract 1 pointfrom the score of each of the other drugs taken by the patient (another cause is more likely) | 0 |
(Interpretation: <0, Very unlikely; 0–1, unlikely; 2–3, possible; 4–5, probable; ≥6, very probable)
Total score in present case=6, means “very probable”
Discussion
Adverse Drug Reactions (ADRs) become a matter of concern as these are one of the leading causes of morbidity and at times mortality among hospitalized patients. Approximately 8% cases among all hospital admissions are due to ADRs as per reports published in different studies [5]. The international classification of SJS/TEN is based on the body surface area (BSA) involved:
Based on BSA insolvent the SCARs are mainly divided into three main categories. By definitions SJS involves <10% of BSA; TEN involves >30%; and overlap syndrome with involvement of 10–30%. [6] Common causes of death include septic shock, hypovolaemic shock, acute renal failure, fulminant hepatitis and multi-organ involvement [6]. Previous studies has shown that most common drugs implicated are sulphonamide antibiotics (38%) and nevirapine (20%) [7]. Antiretrovirals as a group, is important for developing drug induced cutaneous drug reactions [8]. Usually a gap of 4-28 days between beginning of drug use and onset of the adverse reaction is the most suggestive timing for supporting drug causality in SJS or TEN. Study has shown that greatest risk of SCARs with NVP treatment mainly occurred during the first few weeks of treatment initiation [8]. It is the duty of the healthcare provider (s) to counsel patient on HAART therapy regarding early identification and reporting of any kind skin rashes on initiation of therapy.
Diagnosis is challenging as clinical picture ADRs may vary from mild rashes to severe reactions such as SJS or TEN. Toxic Epidermal Necrolysis (TEN) is one of such ADRs which create great threat on public health due to its severe morbidity and mortality. The pathogenesis of SJS/TEN is not fully understood but is believed to be immune-mediated [5]. On histopathology, present case showed extensive keratinocyte apoptosis followed by necrosis. This is the pathogenic basis of the widespread epidermal detachment observed in TEN lesions.
The pathogenesis of SJS/TEN is thought to be a 2-staged process in which the first stage (initiation stage) involves non-inflammatory apoptosis of keratinocytes and the second stage is the amplification phase [9]. In the initiation phase, drug metabolites are thought to mediate CD95 ligand (Fas ligand) and Tumour necrosis factor α (TNF-α) systems stimulating apoptosis. The amplification stage involves inflammatory cells in which TNF-α and IL-2 act as chemotactic cytokines, recruiting CD41 T lymphocytes in the dermis and CD81 cells in the epidermis. During this stage, TNF-α and IL-2 also act as messengers by stimulating the release of chemotactic factors that recruit natural killer lymphocytes and macrophages into the epidermis. The hallmark of the amplification phase of TEN is massive destruction of the epidermis [10].
Nevirapine, a dipyrido-diazepinone, is a Non-Nucleoside Reverse Transcriptase Inhibitor (NNRTI). Nevirapine is an important component of HAART regimen and have been widely used in resource limited countries because of its efficacy, accessibility and comparatively low cost.
The common adverse drug reactions (ADRs) observed with NVP includes skin rashes and hepatotoxicity [11].
Newer pharmacotherapy regimen, specific treatment of opportunistic infections and modified lifestyle has prolonged the life span of HIV infected patients. But adverse drug reactions to Anti-Retroviral Therapy (ART) continue to be a matter of concern, as they inflict significant morbidity and mortality. In present case, the patient suffered severe cutaneous adverse reaction to nevirapine, whose course and morphology gradually evolved from maculopapular rash to targetoid lesions, to extensive erosions of the skin, leading to TEN along with hepatotoxicity and ultimately fatal outcome.
Animal model of NVP induced skin rash demonstrated that 12-hydroxylation metabolic pathway is responsible for developing rash [12]. Previous study it was observed that the CD4+ T cells depletion reduces as well as delays the severity of NVP induced skin rash. Factors may play role in the development and clinical outcome of SJS/TEN are as follows: HIV infection, CD4+ count, causative drug, age, gender, co-medication and use of steroids during the acute stages.
This case report also illustrates the importance of early suspicion of SJS when an HIV-infected patient treated with nevirapine presents with maculopapular rash. The salient features of the earlier published cases have been compared with the present case report in [Table/Fig-7] [13–18].
Salient features of already published cases as compared with the present case report.
| Author | Gender/ Age | Onset of illness (SCARs) | Findings | Outcome |
|---|
| Budamakuntla L et al., [13] | Male/ 44y | 20 days | Patient developed Toxic Epidermal Necrolysis (TEN), leucopenia and hepatotoxicity secondary to intake of nevirapine. | Recovered completely after 1 month |
| Claes P et al., [14] | Male/ 49 y | 13 days | TEN or Lyell’s syndrome, complicated by a toxic hepatitis with severe stomatitis | Recovered completely with a combination of intravenous immunoglobulins and N-acetylcysteine |
| Zingela Z et al., [15] | Male/ 28y | 21 days | Fatal nevirapine-induced Stevens-Johnson syndrome with HIV-associated mania | Stevens-Johnson syndrome (SJS), which progressed to toxic epidermal necrolysis (TEN) and death over a period of 26 days. |
| Patel K et al., [16] | Male/ 46 y | 2 months | Nevirapine and/or co-trimoxazole induced Stevens Johnson syndrome in HIV infected patient | Patient died despite stopping of all medications |
| Mohamed Cissé et al., [17] | Female /28yFemale /26y | 15 days20 days | Two cases of nevirapine induced TEN with fatal outcome in two pregnant women having received it in the context of prevention of mother to child transmission of HIV. | The patients died on Day 9 (D9) and at Day 2 (D2) of hospitalization respectively. |
| Patel IB et al., [18] | Male/ 27 | 10 days | Patient had multiple hemorrhagic lesions present over trunk, upper and lower limb, purpurea present over palms, soles, legs, and toes | Recovered completely |
| Present Case | Male/ 72 y | 25 days | Patient presented to us with a severe maculopapular rash all over the body with targetoid lesions over the limbs with extensive exfoliation involving more than 60 percent of the body surface areas. SCORTEN score was 5. | Died after 7 days of hospital admission |
Patient information, early identification, close observation, timely intervention with meticulous medical and nursing care and support are essential steps for management of TEN. As mortality is high in TEN, clinician must have to be extra cautious in managing HIV infection with ART particularly during the initial two months of treatment to prevent such serious life threatening reactions. A strict vigilance on part of treating physician or health care provider(s) for the initial 2 months is utmost important to prevent such adverse events. Application pharmacogenomics knowledge based on their diverse genetic make-up will define the particular population or a person, who responds differentially to a particular drug.
Conclusion
A 72-year-old male developed TEN secondary to intake of nevirapine. Despite meticulous supportive care and withdrawal of all drugs, his situation worsened and died after 7 days of hospital admission. This simply signifies the chances fatality of nevirapine induced SJS-TEN. For this reason, prompt identification and withdrawal of the culprit drug(s) with early diagnosis, evaluation of the severity and prognosis of disease, rapid initiating supportive care and definite treatment in an appropriate setting is the mainstay for the management of SJS/TEN. Patients should be educated on potential danger symptoms and signs of SJS/TEN when they taking high-risk medicines.
The fatal outcome in this case raises questions regarding the initiation of ART in patients with similar presentations and approach of their management. This case emphasizes the need for further research in Indian patients into these factors to aid clinicians in decision making when it comes to safe options for starting treatment HIV infected patient.
(Interpretation: <0, Very unlikely; 0–1, unlikely; 2–3, possible; 4–5, probable; ≥6, very probable)
Total score in present case=6, means “very probable”
[1]. Bastuji-Garin S, Fouchard N, SCORTEN: a severity-of-illness score for toxic epidermal necrolysisJ Invest Dermatol 2000 115:149-53. [Google Scholar]
[2]. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, A method for estimating the probability of adverse drug reactionsClin Pharmacol Ther 1981 30:239-45. [Google Scholar]
[3]. The use of the WHO-UMC system for standardised case causality assessment. Available from: http://www.WHO-UMC.org/graphics/4409.pdf. (Last accessed on 2014 Sept 30) [Google Scholar]
[4]. Sassolas B, Haddad C, Mockenhaupt M, Dunant A, Liss Y, Bork K, ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: comparison with case-control analysisClin Pharmacol Ther 2010 88:60-68. [Google Scholar]
[5]. Lazarou J, Pomeranz BH, Corey PN, Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studiesJAMA 1998 279:1200-05. [Google Scholar]
[6]. Mockenhaupt M, The current understanding of Stevens-Johnson syndrome and toxic epidermal necrolysisExpert Rev Clin Immunol 2011 7(6):803-15. [Google Scholar]
[7]. Saka B, Barro-Traoré F, Atadokpédé FA, Stevens-Johnson syndrome and toxic epidermal necrolysis in sub-Saharan Africa: A multicentric study in four countriesInt J Dermatol 2013 52(5):575-79. [Google Scholar]
[8]. Paquet P, Jacob E, Damas P, Analytical quantification of the inflammatory cell infiltrate and CD95R expression during treatment of drug-induced toxic epidermal necrolysisArch Dermatol Res 2005 297:266-73. [Google Scholar]
[9]. Sanmarkan AD, Sori T, Thappa DM, Jaisankar TJ, Retrospective analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis over a period of 10 yearsIndian J Dermatol 2011 56:25-29. [Google Scholar]
[10]. Frisch PO, Ruiz-Maldonado R, Erythema multiforme, Stevens Johnson syndrome and toxic epidermal necrolysis. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, editorsFitzpatrick’s dermatology in general medicine 2003 6th ednNew YorkMcGraw-Hill:543-57. [Google Scholar]
[11]. Sabbatani S, Manfredi R, Biagetti C, Chiodo F, Antiretroviral therapy in the real world: population-based pharmacoeconomic analysis of administration of anti-HIV regimens to 990 patientsClin Drug Investig 2005 25:527-35. [Google Scholar]
[12]. Chen X, Tharmanathan T, Mannargudi B, Gou H, Uetrecht JP, A study of the specificity of lymphocytes in nevirapine-induced skin rashJ Pharmacol Exp Ther 2009 331:836-41. [Google Scholar]
[13]. Budamakuntla L, Loganathan E, Suryanarayan S, Abhishek K, Sarvajnamurthy S, Nevirapine induced toxic epidermal necrolysis and non-Hodgkin lymphoma in a Human Immunodeficiency Virus positive patientIndian Dermatol Online J 2014 5:179-81. [Google Scholar]
[14]. Claes P, Wintzen M, Allard S, Simons P, De Coninck A, Lacor P, Nevirapine-induced toxic epidermal necrolysis and toxic hepatitis treated successfully with a combination of intravenous immunoglobulins and N-acetylcysteineEur J Intern Med 2004 15(4):255-58. [Google Scholar]
[15]. Zingela Z, Bronkhorst A, Qwesha WM, Magigaba BP, Fatal nevirapine-induced Stevens-Johnson syndrome with HIV-associated maniaSouthern African Journal of HIV Medicine 2014 15(2):65-66. [Google Scholar]
[16]. Patel K, Panchasara A, Purohit B, Tripathi CB, Nevirapine and/or co-trimoxazole induced Stevens Johnson syndrome in HIV infected patient—a case reportCurr Drug Saf 2013 8(1):72-74. [Google Scholar]
[17]. Cissé M, Soumah MM, Tounkara TM, Diané BF, Sack FB, Baldé H, Lyell Syndrome with Fatal Outcome in Two Pregnant Women Receiving Antiretroviral Therapy in Guinea ConakryJournal of Cosmetics, Dermatological Sciences and Applications 2013 3:135-38. [Google Scholar]
[18]. Patel IB, Adiga S, Shetty DJ, Bairy KL, Nevirapine induced Stevens-Johnson syndrome: a case reportInt J Basic Clin Pharmacol 2014 3:401-02. [Google Scholar]