Baroreflex Dysfunction in Prader Willi Syndrome
Manpreet Kaur1, Shival Srivastav2, Ashok Kumar Jaryal3, Kishore Kumar Deepak4
1 Senior Demonstrator, Department of Physiology, All India Institute of Medical Sciences, New Delhi, India.
2 Junior Demonstrator, Department of Physiology, All India Institute of Medical Sciences, New Delhi, India.
3 Professor, Department of Physiology, All India Institute of Medical Sciences, New Delhi, India.
4 Professor, Department of Physiology, All India Institute of Medical Sciences, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Kishore Kumar Deepak, Room no. 2009, Teaching Block, Second Floor, Department of Physiology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi, India.
E-mail: kkdeepak@gmail.com
Prader-Willi syndrome is a classical hypothalamic insufficiency disorder. This syndrome is often associated with cardiovascular morbidity and mortality - which could probably be attributed to autonomic dysfunction. A 21-year-old Prader Willi syndrome patient was referred for cardiovascular and autonomic function assessment. We performed a battery of tests assessing vascular structure (carotid intima thickness), vascular function (arterial stiffness indices), baroreflex sensitivity (overall integrator of short term regulation of blood pressure), blood pressure variability and autonomic tone (heart rate variability) along with autonomic reactivity tests. We observed impaired baroreflex sensitivity along with orthostatic tachycardia with normal vascular function tests. Prader- Willi syndrome patient have baroreflex dysfunction with probable afferent and/ central autonomic neural defects.
Arterial stiffness, Autonomic dysfunction, Carotid intima thickness
Case Report
A 21-year-old male with Prader Willi Syndrome (PWS) with a body mass index (BMI) of 37.1 kg/m2 and an overall intelligence quotient (IQ) of 65-69 (mild mental retardation) was referred to Cardiovascular and Autonomic function lab at Department of Physiology, All India Institute of Medical Sciences, New Delhi. Patient had a history of progressive weight gain, delayed secondary sexual characteristics, glycosuria and was diagnosed as a case of type 2 diabetes mellitus.
Standard battery of autonomic function tests – head-up tilt (HUT), deep breathing test (DBT), Valsalva’s manoeuvre (VM), isometric exercise pressor test (IET) and cold pressor test (CPT) were performed after a period of 15 minutes supine rest [1]. Continuous beat to beat blood pressure and lead II ECG were recorded using Finapres‡ and further analysis was done using LabChart Pro‡ Version 7.0.
The basal blood pressure of the patient was 132/68 mm Hg with a heart rate of 88 beats per minute. During head-up tilt (HUT) manoeuvre, patient had a fall of 26/15 mm Hg in systolic and diastolic blood pressure at 0.5 min along with a rise of 23 beats/min in heart rate. Blood pressure returned to 132/64 mm Hg at 0.75 min and was maintained thereon for 5 minutes period with a higher heart rate of 118 beats/min (delta heart rate = 30 beats/min). A 30:15 ratio of one was obtained. Thus the findings of HUT were suggestive of initial fall in BP with recovery at the cost of orthostatic tachycardia with abnormal 30:15 ratio.
Deep breathing test (E:I ratio = 1.3 and delta HR= 17) was within normal limits. On performing, isometric exercise pressor test and cold pressor test, the patient had a rise of 21 and 12 mm Hg diastolic blood pressure respectively and thus a normal response. Valsalva Manoeuvre could not be performed by the patient.
Heart rate variability parameters of both time domain {SDNN=33.18 ms, SDSD=14.82 ms and RMSSD=14.80 ms} and frequency domain {Low Frequency (LF) power=237.95 ms2, High Frequency (HF) power=29.27 ms2 and Total power (TP) =1130.13 ms2} were within normal limits; however the LF/HF ratio of 6.05 was observed.
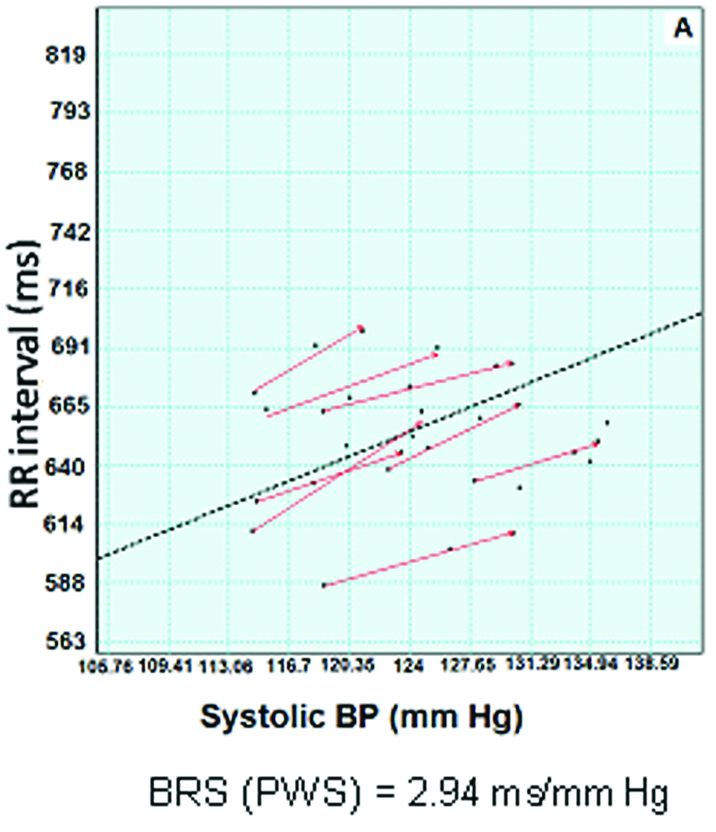
Spontaneous baroreflex sensitivity (BRS) was assessed by 5 minutes simultaneous basal recording of blood pressure and lead II ECG using Finapres‡ by sequence and spectral method using Nevrokard‡. Baroreflex sensitivity (systolic BP) - determined by sequence method {2.94 ms/mm Hg} [Table/Fig-1] and spectral method {alpha (LF) =3.7128 and alpha (HF) =1.5634} -was found to be impaired as compared to normal data for this age group [2].
Depicts systolic baroreflex sensitivity of prader-Willi syndrome patient. dotted line indicates the slope(baroreflex sensitivity). X axis denotes blood pressure in millimeter of mercury (mmHg) and Y axis denotes RR interval in milliseconds (ms).
Arterial stiffness indices- carotid-radial pulse wave velocity and augmentation index (AI) were 6.6 m/s and 1% respectively (within normal limits).
Carotid-Intima Media Thickness (CIMT) assessed using vascular B Mode ultrasound (anterior = 0.44 cm and posterior=0.41 cm) was found to be normal.
Discussion
Cardiovascular events in Prader-Willi syndrome patients are a common occurrence. This could be attributed to baroreflex dysfunction observed in these patients. Baroreflex sensitivity is an indicator of baroreflex loop function. Baroreflex sensitivity (BRS) has shown to be an independent predictor of cardiovascular mortality. Impairment in baroreflex function in these patients could be principally due to a vascular defect owing to the metabolic profile of these patients – obesity, diabetes and insulin resistance or neural/autonomic defect due to either involvement of higher neural input from hypothalamus to the baroreflex loop or simply a brainstem defect itself. Autonomic dysfunction has been observed in Prader- Willi syndrome patients [3]. The question remains - What is the probable mechanism of baroreflex dysfunction in PWS patients - is it purely neural or vascular or a multilevel involvement?
To the best of our knowledge, till date, no studies have addressed this issue with a comprehensive multi-level assessment. The findings of the present case-possibly throw some light in this direction to understand the principal site of involvement in PWS patients.
In the present case of PWS, we found impaired baroreflex sensitivity with orthostatic tachycardia diagnosed as per the current literature [4].
Interestingly, vascular structure and function as assessed by Carotid-Intima Media Thickness (CIMT) and arterial stiffness indices (pulse wave velocity and augmentation index) were found to be normal. Our results were in concordance with the available previous literature [5]. This supports the idea that reduction in baroreflex sensitivty in PWS is not attributable to vascular dysfunction.
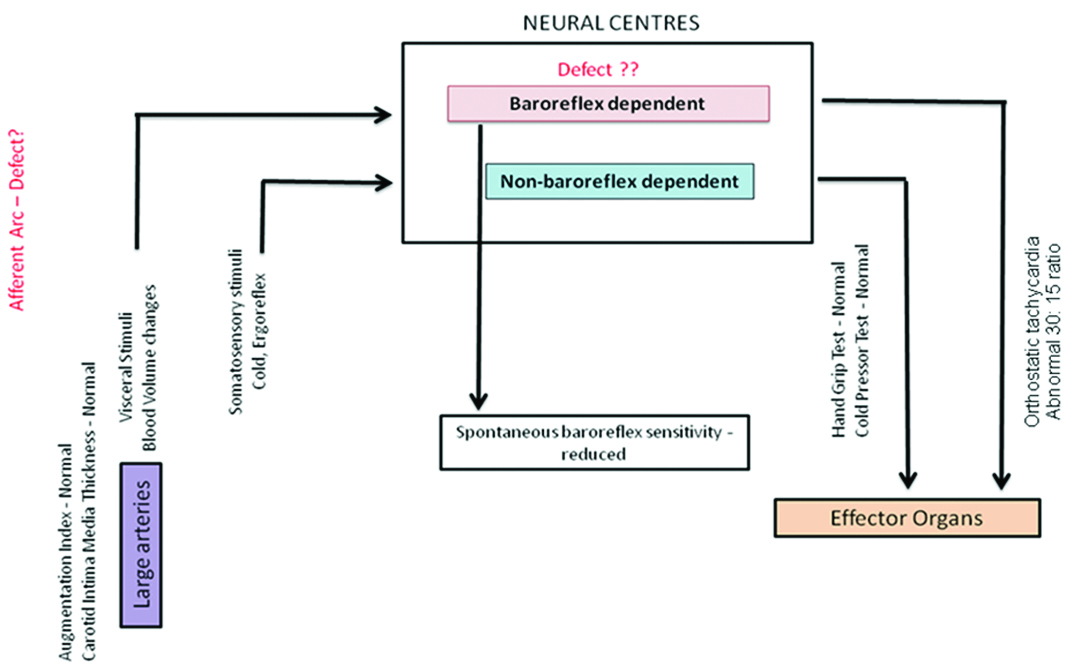
The strength of our case report is that we used cardiac autonomic reactivity tests. Autonomic reactivity tests assess the ability of the body to respond to various physiological stimuli. These tests can be classified broadly into baroreflex dependent (head-up tilt and Valsalva’s manoeuvre) and non-baroreflex dependent tests (cold pressor test and isometric exercise pressor test). Baroreflex dependent tests essentially evaluate the baroreflex loop integrity and non-baroreflex dependent tests study the response to somatosensory stimuli (cold etc.) and the sympathetic efferent arc integrity. Sympathetic efferent arc is common to both baroreflex dependent and non-dependent loop. In our study, we found a normal response to cold pressor and isometric exercise pressor test (non-baroreflex dependent loop) along with orthostatic tachycardia with abnormal 30:15 ratio (baroreflex-dependent loop). Orthostatic tachycardia could be a sign of impending baroreflex failure [6]. The above results suggest that non-baroreflex dependent pathways are normal suggestive of a normal efferent arc while we do have a defect in baroreflex-dependent pathway. Thus the baroreflex dysfunction observed in this patient could probably be due to an afferent or central autonomic neural defect.
Our results are in concordance with a similar study on Prader- Willi syndrome patients [7] where they found impaired postprandial autonomic nervous system responsiveness in PWS patients with no significant differences in arterial stiffness indices as compared to BMI matched obese and lean subjects.
This case report is relevant and instructive as it can explain the possible mechanism of cardiovascular dysfunction in PWS syndrome [Table/Fig-2].
Baroreflex loop dysfunction with a normal efferent arc suggestive of a probable pathological involvement at the level of neural centers and afferent arc.
Limitation of the present case report is the assessment of parameters in a single subject. Future studies could be designed to perform comprehensive assessment of a considerable number of PWS patients and thus test the theoretical model proposed here and decipher the underlying patho-physiology of the disorder.
Conclusion
There is baroreflex dysfunction in PWS patients with normal vascular component and principally neural involvement – where also the efferent arc is essentially normal with probable defect in afferent or central autonomic neural defect.
The authors declare that they have no conflict of interest.
[1]. Hohnloser SH, Klingenheben T, Basic autonomic tests. In: Malik M, editor. Clinical guide to cardiac autonomic tests 1998 NetherlandsKluwer Academic Publishers:51-65. [Google Scholar]
[2]. Tank J, Baevski RM, Fender A, Baevski AR, Graves KF, Ploewka K, Reference values of indices of spontaneous baroreceptor reflex sensitivityAm J Hypertens 2000 13(3):268-75. [Google Scholar]
[3]. DiMario FJ, Dunham B, Burleson JA, Moskovitz J, Cassidy SB, An evaluation of autonomic nervous system function in patients with Prader-Willi syndromePediatrics 1994 93(1):76-81. [Google Scholar]
[4]. Grubb BP, Postural tachycardia syndromeCirculation 2008 117(21):2814-17. [Google Scholar]
[5]. Patel S, Harmer JA, Loughnan G, Skilton MR, Steinbeck K, Celermajer DS, Characteristics of cardiac and vascular structure and function in Prader-Willi syndromeClin Endocrinol (Oxf) 2007 66(6):771-77. [Google Scholar]
[6]. Ketch T, Biaggioni I, Robertson R, Robertson D, Four faces of baroreflex failure: hypertensive crisis, volatile hypertension, orthostatic tachycardia, and malignant vagotoniaCirculation 2002 105(21):2518-23. [Google Scholar]
[7]. Purtell L, Jenkins A, Viardot A, Herzog H, Sainsbury A, Smith A, Postprandial cardiac autonomic function in Prader-Willi syndromeClin Endocrinol (Oxf) 2013 79(1):128-33. [Google Scholar]