Dental professionals are exposed to a wide variety of microorganisms. The use of effective infection control procedures in the dental office and dental laboratory can prevent cross-contamination to dentists, dental office staff, dental technicians and patients [1]. Concern about dissemination of these organisms has produced renewed interest in denture sterilization and disinfection [2]. Heat polymerized dentures exhibit dimensional change during disinfection procedure [3].
Fabrication of dental prosthesis using heat-cured denture acrylic resin is one of the commonest treatment procedure in all the dental offices [1]. Infection control has become a necessity to protect dentist, staff and patients [4]. Considering the cross-contamination between the dental operatory and dental laboratory, dental prostheses must be disinfected before sending to the laboratory and before delivery to the patient [5].
Various methods of sterilization and disinfection in the dental office have been suggested. The guidelines describe the four chief categories of disinfectants that are accepted by the Council on Dental Therapeutics. These are chlorine solutions, formaldehyde, glutaraldehyde and iodophors [5]. Alternative methods for disinfection and sterilization of dental prostheses are microwave irradiation [6,7] and Photodynamic Therapy (PDT) [8]. In-vitro studies on microwave irradiation has given conflicting results because when an acrylic resin is subjected to temperature greater than 71oC, the material can undergo plastic deformation [6]. Photodynamic disinfection of dentures is based on photochemical action of photoactive dye (photosensitizers), activated by proper light in the presence of oxygen [8]. However, despite its effectiveness, there is lack of literature on the effect of PDT disinfection protocol on the physical and mechanical properties of the denture materials.
One of the safest and easiest method is to disinfect the prosthesis by immersing in chemical disinfectants [9]. But studies have shown that heat-cured acrylic specimens exhibit dimensional change during disinfection procedure [3,9,10]. In evaluating the ideal denture-base material, one important comparison that is of concern is dimensional stability of the denture-base after immersion [10,11]. The purpose of this study was to evaluate and compare the effect of commonly used laboratory disinfecting agents on the dimensional stability of different commercially available heat-cured denture acrylic resins in India.
Materials and Methods
This in-vitro study was conducted in the Department of Prosthodontics, College of Dental Sciences, Davangere. A smooth circular metal disc [Table/Fig-1] made up of steel measuring 36mm x 3mm was machined to maintain standard dimension of acrylic specimens. A very thin layer of Vaseline is applied onto the surface of metal disc for ease of removal. The disc was invested in type III dental stone (Kal Stone, Kala Bhai Kursan Pvt. Ltd.) in the conventional manner. After final setting time two halves of the standard brass processing flask (KAVO, Kavo dental (India) Pvt. Ltd.) were separated and the metal disc was taken out. Stone mold was thoroughly washed, allowed to dry and very thin layer of cold mould seal (Dental Products of India Ltd.) was applied and allowed to dry.
Circular metal disc used to prepare acrylic specimens.

Three heat-cured denture acrylic resins [Table/Fig-2] commercially available in the Indian market and popularly used by dental profession namely Acralyn-H (ASIAN Acrylates), Trevalon (DENTSPLY India Pvt. Ltd.) and Stellon (Dental Products of India Ltd.) were used to prepare acrylic specimens. As per the manufacturer’s instructions, the monomer and polymer ratios were added. The resin was packed at dough stage into the flask and graduated pressure to the extent of 3000psi by using hydraulic press was applied. After three trial closures for each specimen the flasks were transferred to the clamp for bench curing. The manufacturer’s instructions for curing were meticulously followed for comparison and accuracy of results. All the specimens were trimmed, only to remove excess flash remaining after three trial closures, without altering the original dimension to maintain uniform size of all the specimens and polished to luster only on one side to resemble denture base. Twelve such specimens each of three different brands were prepared and systematically labeled for easy identification [Table/Fig-3].
Resins used in this study.
Resin specimens of three different brands.
Diameter of the specimens was accurately measured by using Profile Projector (Nikon V-12) prior to chemical immersion. The circular disc was marked at three points and three measurements made and average was taken to eliminate any error. Thus, a total of 36 specimens were measured (H0). Twelve specimens each of three different brands were immersed in three chemical disinfectants namely 2% alkaline glutaraldehyde (Cidex, Jalagaon Chemical Pvt. Ltd.), povidone- iodine 0.5% (Betadine, G.S. Pharmabutor Pvt., Ltd.), sodium hypochlorite solution (3D Fine-Chem Ltd.) [Table/Fig-4] and water which acts as a control group. All the three disinfectant solutions were placed in three different glass containers and water in another glass container. Three specimens each of different brands were immersed in all the four containers [Table/Fig-5] for 1 hour and 12 hours and subsequently measurements were made after 1 hour (H1) and after 12 hours (H2) as shown in [Table/Fig-6,7]. All tests were carried out at room temperature (280C) and in dry environment.
Disinfectants used in this study.
Acrylic specimens immersed in three disinfectants and water.

Comprehensive tabulation of dimensions of specimens in three disinfectants and water before (HO) and after 1 hour (H1) and 12 hours (H2) of immersion.
| Types of Resins | No. of Specimens | DIAMETER OF SPECIMENS (mm) |
|---|
| 2% AlkalineGlutaraldehyde | 1% SodiumHypochlorite | 0.5% Povidone-Iodine | Water |
|---|
| Duration of Immersion | Duration of Immersion | Duration of Immersion | Duration of Immersion |
|---|
| HO | H1 | H2 | H0 | H1 | H2 | H0 | H1 | H2 | HO | H1 | H2 |
|---|
| Acralyn | 1 | 35.651 | 35.659 | 35.677 | 35.677 | 35.686 | 35.703 | 35.675 | 35.681 | 35.691 | 35.672 | 35.680 | 35.713 |
| 2 | 35.688 | 35.694 | 35.722 | 35.644 | 35.651 | 35.687 | 35.665 | 35.673 | 35.689 | 35.647 | 35.649 | 35.779 |
| 3 | 36.692 | 35.695 | 35.718 | 35.659 | 35.666 | 35.686 | 35.657 | 35.658 | 35.695 | 35.691 | 35.691 | 35.730 |
| Stellon | 4 | 35.748 | 35.755 | 35.769 | 35.761 | 35.769 | 35.780 | 35.692 | 35.694 | 35.721 | 35.689 | 35.706 | 35.724 |
| 5 | 35.697 | 35.701 | 35.727 | 35.762 | 35.766 | 35.793 | 35.732 | 35.739 | 35.755 | 35.727 | 35.733 | 35.762 |
| 6 | 35.714 | 35.721 | 35.731 | 35.697 | 35.707 | 35.731 | 35.741 | 35.745 | 35.775 | 35.749 | 35.760 | 35.780 |
| Trevalon | 7 | 35.813 | 35.814 | 35.838 | 35.878 | 35.883 | 35.899 | 35.804 | 35.813 | 35.822 | 35.787 | 35.794 | 35.819 |
| 8 | 35.869 | 35.875 | 35.888 | 35.835 | 35.843 | 35.873 | 35.831 | 35.837 | 35.856 | 35.826 | 35.835 | 35.854 |
| 9 | 35.815 | 35.816 | 35.825 | 35.777 | 35.780 | 35.798 | 35.847 | 35.856 | 35.866 | 35.840 | 35.851 | 35.871 |
Percentage linear changes and their comparison between different disinfectants.
| Disinfectants | Particulars | H0 | H1 | Difference | H2 | Difference |
|---|
| H0-H1 | % | H2-H0 | % |
|---|
| 2% AlkalineGlutaraldehyde (n=9) | Mean± SD | 35.743±0.073 | 35.748± 0.072 | 0.005±0.003 | 0.013 ± 0.007 | 35.766±0.069 | 0.023±0.007 | 0.065±0.020 |
| 1% Sodium Hypochlorite (n=9) | Mean± SD | 35.726± 0.027 | 35.733± 0.027 | 0.007± 0.004 | 0.020 ± 0.011 | 35.754± 0.026 | 0.028±0.007 | 0.079±0.018 |
| 0.5% Povidone-Iodine (n=9) | Mean± SD | 35.827± 0.030 | 35.833± 0.031 | 0.006±0.003 | 0.017 ± 0.09 | 35.851± 0.031 | 0.024±0.008 | 0.067±0.021 |
| Water (n=9) | Mean± SD | 35.736± 0.069 | 35.745± 0.070 | 0.009±0.004 | 0.025 ± 0.012 | 35.781± 0.057 | 0.045±0.033 | 0.126±0.092 |
| Difference between Disinfectants | F=2.53 p=0.06 | F=2.78 p=0.06, NS |
All the specimens were evaluated for change in diameter. The co-efficient of variation never exceeded 0.1%. The percentage difference between H0, H1 & H2 were statistically analyzed. One way analysis of variance was used for assessing the dimensional changes.
Results
The measurements of diameter of all the 36 acrylic specimens were made at three different stages namely before immersion (H0), after 1 hour immersion (H1) and after 12 hours of immersion (H2) in chemical disinfectants and water are shown in [Tables/Fig-6] . The mean, the standard deviation, amount of dimensional change, the percentage linear changes and their comparison between different disinfectants are listed in [Table/Fig-7]. The mean, the standard deviation, the percentage linear changes and their comparison between different brands of resins before immersion (H0), after 1 hour immersion (H1) and after 12 hours of immersion (H2) are presented in [Table/Fig-8].
Percentage linear changes and their comparison between different brands of resins.
| Disinfectants | Particulars | H0 | H1 | Difference | H2 | Difference |
|---|
| H0-H1 | % | H2-H0 | % |
|---|
| Acralyn-H(n=12) | Mean± SD | 35.668± 0.017 | 35.676± 0.018 | 0.008±0.003 | 0.022± 0.007 | 35.708±0.028 | 0.039±0.030 | 0.110±0.085 |
| Stellon(n=12) | Mean± SD | 35.726± 0.027 | 35.733± 0.027 | 0.007±0.004 | 0.020± 0.011 | 35.754± 0.026 | 0.028±0.007 | 0.079±0.018 |
| Trevalon(n-12) | Mean± SD | 35.827± 0.030 | 35.833± 0.031 | 0.006±0.003 | 0.017± 0.09 | 35.851± 0.031 | 0.024±0.008 | 0.067±0.021 |
| Difference between Disinfectants | F=0.43 p=0.66, NS | F=2.26 p=0.12, NS |
All the specimens in three disinfectants as well as in water exhibited very small amount of linear expansion after 1 hour and 12 hours of immersion. Among three disinfectants, specimens in 2% alkaline glutaraldehyde exhibited least amount of dimensional change of 0.005mm and 0.065mm followed by 0.5% povidone iodine i.e., 0.006mm and 0.070mm and 1% sodium hypochlorite i.e., 0.007mm and 0.081mm after 1 hour and 12 hours of immersion respectively. The poorest dimensional stability was exhibited by specimens in water with linear changes of 0.009mm and 0.126mm after 1 hour and 12 hours respectively. Statistical analysis of the data, however, revealed that difference in dimensional changes were not statistically significant (p>0.05).
All the three brands of resins exhibited very small amount of linear expansion in all the disinfectants and water after 1 hour and 12 hours of immersion. Trevalon specimen showed least amount of dimensional change of 0.006mm and 0.067mm followed by Stellon 0.007mm and 0.079mm and Acralyn-H 0.008mm and 0.110mm after 1 hour and 12hours of immersion respectively. Statistical analysis of the data revealed that the difference in the dimensional changes between Trevalon, Stellon and Acralyn-H were statistically insignificant (p>0.05).
Discussion
Prostheses contaminated with pathogenic microorganisms serve as a potential source of infection transmission between patients and dental personnel. To prevent cross-contamination, prostheses should be completely disinfected before being sent to the laboratory and before insertion. Several methods of disinfection have been recommended to ensure infection control in dental practice [2]. As they cannot be exposed to high temperature, the safest, convenient and inexpensive method to disinfect the prostheses is by immersing in chemical disinfectants [5]. The commonly recommended chemical disinfectants for removable dental prostheses are 2% alkaline glutaraldehyde, iodophors and 1% sodium hypochlorite [5].
The effect of disinfectants on physical and mechanical properties of denture resins should also be considered during disinfection. Dimensional stability was critical for our study because heat-cured acrylic specimens exhibit dimensional change during disinfection procedure [12,13]. M. Braden and Craig reported that sorption takes place when dentures are stored in water or in contact with oral fluids resulting in dimensional change [14,15]. The present study was standardized by taking pink veined acrylic in all the three brands of resins, metal disc was used to maintain uniform size of specimens and polishing done only on one side to resemble a denture base.
Comparison of chemical disinfectants: The specimens were subjected to an immersion time of 0 hour, 1 hour and 12 hours. From the study it was observed that all the disinfectants including water, induced linear expansion of the specimens. However, disinfectants 2% alkaline glutaraldehyde induced least dimensional change followed by 0.5% povidone iodine, 1% sodium hypochlorite and water induced highest [Table/Fig-6]. Percentage linear changes between three disinfectants and water were found to be statistically insignificant (p>0.05).
The present study was in agreement with the results reported by Polyzoise et al., who reported that heat cured acrylic specimens exhibited negligible dimensional change after disinfection procedure and were not of clinical importance [3] and findings by David M. Bohnekamp, who concluded that acrylic resins increase in dimension on wetting [16]. The plastic denture base materials have a tendency for high degree of water sorption [15]. A high percentage of water sorption is associated with serious warpage and dimensional change in the material [15]. Acrylic resins increase in dimension on wetting [17]. According to M Braden, absorption process depends on two physical parameters namely, equilibrium water absorption and diffusion co-efficient [14].
In the present study, the highest value of diffusion was observed for specimens in water 0.126% and lowest value of 0.065% for specimens in 2% alkaline glutaraldehyde. This may be due to following reasons: a) Molecular weight of water is very low compared to disinfectants [18]. According to Graham’s law of diffusion, molecular weight is inversely proportional to diffusion [19]; b) Water is a highly polar molecule [20]. But 2% alkaline glutaraldehyde is an organic compound and they are non-polar molecules [21]. The absorption is undoubtedly primarily due to the polar properties of the resin molecules [22]; c) Viscosity of liquids (disinfectants and water) were measured using Viscometer [Table/Fig-9]. Viscosity of water is low compared to other disinfectants. As the viscosity of the substance is inversely proportional to its diffusion [23], the rate of diffusion of water is higher than disinfectants; d) The pH of the liquids were measured using pH meter [Table/Fig-10]. According to Craig, resistance of polymethyl methacrylate to weak acids and bases is good [15]. Hence pH nearer to neutral may be favoring diffusion into acrylic resin, which is water. Therefore, diffusion of water could be more compared to disinfectants; e) Acrylic resin is a polar polymer and water is a highly polar molecule [20]. Therefore, when two polar molecules interact, the rate of diffusion is more; f) 2% alkaline glutaraldehyde is a weak organic electrolyte sparingly soluble in water [23]. Hence, the solubility parameter of it is not matching with acrylic resin. Therefore, the rate of penetration of 2% alkaline glutaraldehyde into a polar acrylic resin molecule is very less.
Viscosity of different solutions used in the study.
| Name of the disinfectant | Viscosity (in kg/m.s) |
|---|
| Water | 0.0875 X 10-3 |
| 1% Sodium Hypochlorite | 0.973 X 10-3 |
| 0.5% Povidone-Iodine | 1.418 X 10-3 |
| 2% Alkaline Glutaraldehyde | 3.182 X 10-3 |
pH of different solutions used in the study.
| Name of the solutions | pH |
|---|
| Water | 6.8 |
| 1% Sodium Hypochlorite | 8.4 |
| 0.5% Povidone-Iodine | 5.15 |
| 2% Alkaline Glutaraldehyde | 9.02 |
Among the remaining two disinfectants, 1% sodium hypochlorite shows higher diffusion than 0.5% povidone-iodine solution. This may be due to following reasons: a) Chlorine molecule posses lower molecular volume than iodine [11]; b) The pH of sodium hypochlorite (8.4) is more nearer to neutral compared to 0.5% povidone-iodine; c) Viscosity of 1% sodium hypochlorite is less compared to that of 0.5% povidone iodine.
Comparison of Resins: Statistical scrutiny of the values, revealed that the percentage linear changes of different brands of resins were not statistically significant (p>0.05). Trevalon was found to be more dimensionally stable compared to Stellon and Acralyn-H. This variation may be due to:
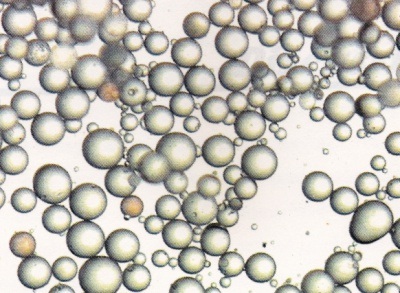
1. Acrylic resins used in this study are synthesized by suspension polymerization or pearl or bead polymerization [12]. Higher the molecular weight, higher the degree of polymerization [15]. Larger the size of the beads more will be the dimensional stability [12,17]. Low molecular weight particles get fully solvated and then diffuse out quickly while higher molecular weight particles are still in the mode of progressive solvation and need a very longer time to diffuse out [24,25]. This result is supported by the stereomicroscopic study of size of the acrylic beads [Table/Fig-11,12,13 and 14]. It indicates Trevalon is having larger sized particles followed by Stellon having medium sized particles and Acralyn-H having smallest particles. This stereomicroscopic observation is in agreement with the findings reported by Craig [15]. In the present study, the sequence of dimensional change for different resins is in agreement with the size of the beads i.e, larger the size of the beads smaller will be the dimensional change.
Size of the resin particles of different brands used in the study.
| Name of the resin | Range of particle size (in μm) | Average particle size |
|---|
| Acralyn-H | 25.729-80.002 | 47.714 |
| Stellon | 37.556-91.710 | 54.250 |
| Trevalon | 69.037-109.632 | 79.700 |
Stereomicroscopic appearance of Acralyn-H particles.

Stereomicroscopic appearance of Stellon particles.
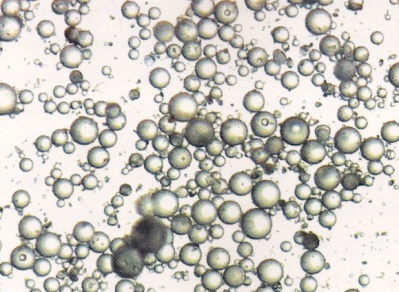
Stereomicroscopic appearance of Trevalon particles.
2. Mismatching additives like coloring agents present in the polymer may disturb the well ordered structure of the polymer and hence increase in the rate of diffusion resulting in more dimensional change [10, 25–27].
3. If the additives added increase the secondary forces (dipole-dipole interaction and hydrogen bond formation) between polymer to polymer layers, reduces the diffusion behavior [27].
Limitation
It is suggested that further investigation be accomplished to simulate the study clinically by conducting the same on the clinical dentures which would give us a more accurate finding since the present study is limited to circular acrylic discs. Furthermore, as this study is limited to only three brands of denture acrylic resins, the results obtained may not be applicable to other brands. There is scope for research on different brands of denture acrylic resins commercially available in India.
Conclusion
Within the limitations of this study, the following conclusion can be drawn:
All the specimens of different brands exhibited increase in the linear dimensional change in all the disinfectants as well as in water.
Of the three disinfectants used, specimens immersed for 1 hour and 12 hours in 2% alkaline glutaraldehyde showed least amount of dimensional change followed by 1% sodium hypochlorite and 0.5% povidone-iodine and in water showed highest amount of dimensional change.
Of the three commercially available heat-cured denture acrylic resins tested, Trevalon exhibited least dimensional change followed by Stellon. Acralyn-H showed highest dimensional change.
Dimensional changes observed between different resins and different disinfectants were statistically insignificant.