Chronic Liver Disease (CLD) is a pathological state of the liver characterised by gradual destruction of liver tissue over time. Alcoholic cirrhosis, non-alcoholic fatty steatosis and hepatitis, mainly hepatitis B and C are the most common cause for CLD and are an important cause of morbidity and mortality worldwide [1]. The crude mortality rate of CLD was 40 per 100,000 population [2]. Albumin is a major plasma protein that is synthesized primarily in the liver. Serum concentration of albumin is reduced in patients with chronic liver failure [3]. Albumin plays an important functional roles like transport of hormone, ligands and drugs and any changes to it which quantitatively and qualitatively will affect the disease course and treatment outcomes [4]. It is thus imperative to investigate on this crucial protein so as to improve the management of patients with CLD.
Ischemia Modified Albumin (IMA) is an upcoming biomarker that has been shown to be elevated in various diseases associated with ischemia and oxidative stress like Myocardial Infarction, Diabetes Mellitus, Renal Failure, pulmonary embolism, Cerebrovascular accidents, sepsis etc. It is seen that oxidative stress causes a conformational change at the N terminal end of the albumin molecule. The metallic ion, which is copper or cobalt normally, binds to the N terminal end of the albumin molecule. Due to conformational change, albumin fails to do so and this modified form of albumin is referred to as IMA. The exact mechanism that leads to the formation of IMA is not known. Studies have shown that IMA can be used as a ubiquitous indicator for oxidative stress and ischemic damage [5–9].
Patients with CLD are often plagued by ischemia and free radical damage. Oxidative stress is central to the pathogenesis of CLD and more so with alcoholic liver disease. Decompensation further accentuates the production of Reactive Oxygen Species. The production of free radicals has been attributed to the derangement of metabolic functions of the liver. It has also been hypothesised that excess amounts of oxidative agents are released from the mitochondria upon injury to the liver [10]. Since IMA gives a measure of the overall level of ischemic damage and oxidative stress in the body [7], estimation of IMA can aid to quantify oxidative stress in patients with CLD. This will give an idea regarding the severity of the disease and prognosis of patients and can aid in determining the level of care needed. It may also aid to quantify the extent of liver injury.
The current study estimates the serum level of IMA and IMA/Albumin ratio in patients with CLD and correlate both with the various parameters of liver function tests (LFT) which includes albumin, bilirubin, aspartate transaminase (AST), alanine transaminase (ALT), Gamma Glutamyl Transpeptidase (GGT) and Alkaline phosphatase (ALP) and Prothrombin Time (PT) to see if IMA can be used to assess the severity of hepatic damage. Correlation was also done with the Model for End stage Liver Disease (MELD) score, a scoring system that determines the short term mortality in CLD patients to see if IMA can predict mortality and thus denote outcome in such patients.
Materials and Methods
This study was conducted after obtaining ethical approval from institutional Human Ethics Committee. The study period was for six months from June to November 2014. Total 43 consecutive patients with CLD and 28 apparently healthy human volunteers as controls participated in the study. Patients who were clinically diagnosed as CLD by a certified gastroenterologist from the Department of Gastroenterology were recruited for the study within 24 hours of admission. Patients with clinical conditions which were known to alter serum IMA levels namely, renal disease, diabetes mellitus, heart failure, sepsis, pulmonary embolism, hyperlipidaemia and cerebrovascular accidents [5–9] and those with extremes of age (below 18 or above 80 years) were excluded. Control group included people who showed no clinical signs of any disease undergoing routine check-up.
A 5 mL of venous blood sample was collected from the subjects and serum was separated. Serum samples were stored at -20oC if not processed immediately. Citrated plasma was used for prothrombin time measurement and the samples were processed immediately.
Albumin, bilirubin, ALT, AST, GGT, ALP and creatinine were estimated using the automated analyser Cobas 6000 [11] in the Biochemistry laboratory. The instrument takes an aliquot of the patients sample and dilutes it with normal saline. It uses the colorimetric principle and certain formulas to measure the parameters automatically [11]. PT INR was measured using the viscosity based clot detection system by stago [12] in the clinical pathology laboratory.
Serum IMA was estimated using the Albumin Cobalt Binding Test (ABT). The test is based on the principle that a sample containing IMA will bind less cobalt and hence will have more free cobalt ions. Measuring the free cobalt is an indirect method to quantify IMA. To 200 μL of patients serum 50 μL of cobalt chloride solution (1 g/L) was added followed by vigorous mixing and incubated for 10 minutes at room temperature. 50 μL of Dithiothretol solution (1.5 g/L) was added followed by vigorous mixing and 2 minutes of incubation in room temperature. Dithiothretol binds to cobalt and thus is a selective indicator of free cobalt. The last step involved adding 1 mL of normal saline to the sample. The blank was prepared similarly by excluding Dithiothretol. The solution was read in a Gilford spectrometer at 470 nm and IMA values were recorded in Absorbance units (ABSU) [7].
Albumin and IMA were estimated for both the test and control groups. All other parameters were check only on the test group patients as they were established markers used in routine clinical practice and comparison with control group was not needed.
The MELD score was calculated using the formula: 3.8{loge serum bilirubin (mg/dL)} + 11.2 {loge INR} + 9.6 {loge serum creatinine (mg/dL)} + 6.4. Any patient dialysed twice in the previous week was given a creatinine value of 4. A value less than 1 was taken as 1 [13].
Statistical Analysis
Statistical analysis was done using Medcalc software Version 12.7. The type of data was parametric. Student t-test was used to compare the levels IMA and IMA/Albumin ratio of the test and control groups. A p-value of < 0.05 was considered significant. Correlation coefficient test was used to analyse the correlation between the various parameters done in the study and the r and p-values were determined. Tables and graphs were used to express the data.
Results
The study was conducted on 43 patients with CLD in the age range of 25 to 80 years comprising of 36 males and 7 females. The control group had 28 apparently healthy adults in the age range of 28 to 70 years of which 13 were males and 15 were females.
[Table/Fig-1] shows the mean values of serum albumin, IMA and IMA/Albumin ratio along with the respective p-values.
Table representing the mean values of the serum level of Albumin, IMA and IMA/Albumin ratio in CLD patients and the control group.
| Parameter | Patients with CLD | Control group | p-value |
|---|
| Serum Albumin (g/dL) | 2.8977 ± 0.7661 | 4.5143 ± 0.2663 | < 0.0001 |
| Serum IMA (ABSU) | 0.5320 ± 0.1677 | 0.3203 ± 0.1257 | < 0.0001 |
| IMA/Albumin Ratio | 0.2035 ± 0.0970 | 0.0714 ± 0.0283 | < 0.0001 |
Bilirubin, ALT, AST, ALP, GGT, creatinine and PT INR were estimated for the test group alone and their mean values are represented along with the standard reference range in [Table/Fig-2] [14–17].
Table representing the mean values of various liver function parameters analysed in CLD patients.
| Parameter | Mean value | Normal range |
|---|
| Bilirubin (mg/dL) | 5.0395±4.6456 | 0.1-1.2 [16] |
| ALT (IU/L) | 30.8333±20.4904 | 7-56 [13] |
| AST (IU/L) | 57.9706±39.5179 | 0-35 [13] |
| ALP (IU/L) | 125.6053±64.2224 | 41-133 [13] |
| GGT (IU/L) | 57.0588±47.3273 | 9-85 [13] |
| Creatinine (mg/dL) | 1.3930±1.2175 | 0.6-1.5 [17] |
| PT INR | 1.8620±0.7080 | 0.8-1.2 [18] |
The level of serum albumin was significantly low in patients with CLD compared to normal controls. Serum IMA was significantly higher in patients with CLD compared to control group {0.5320 ± 0.1677 vs 0.3203 ± 0.1257} with a p-value of < 0.0001. IMA/Albumin ratio was also significantly higher in patients with CLD than the control group {0.2035± 0.0970 vs 0.0714 ± 0.0283} with a p-value of < 0.0001.
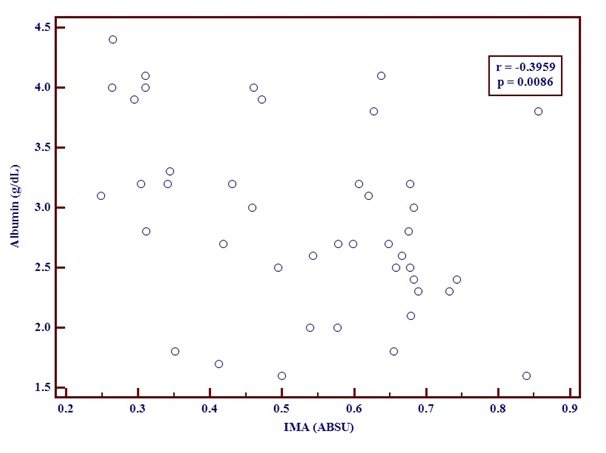
The correlation between Serum albumin and IMA levels [Table/Fig-3] in CLD patients was done. The correlation coefficient r was -0.3959 with a p-value of 0.0086. This demonstrates that IMA and Albumin in patients with Chronic liver disease have an inverse relationship.
Graph showing negative correlation between serum albumin and IMA using correlation coefficient test.
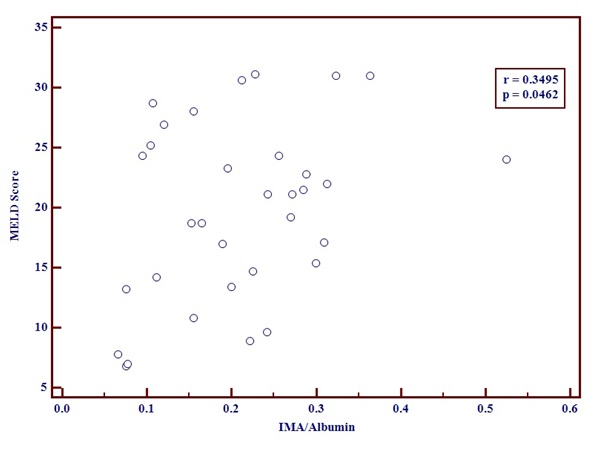
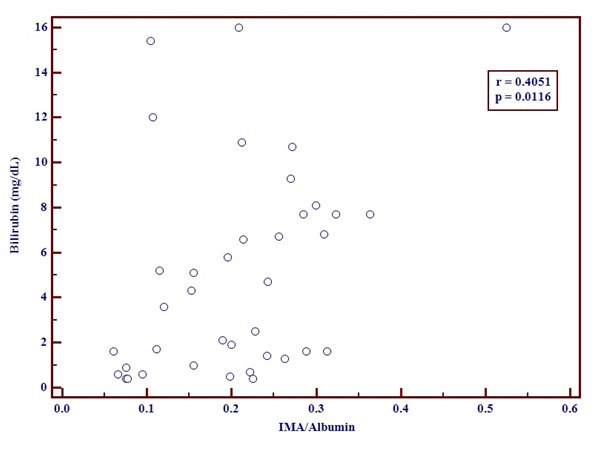
The IMA/Albumin ratio and MELD score [Table/Fig-4] showed a positive correlation. The correlation coefficient r was 0.3495 with a p-value of 0.0462.A positive correlation was seen between IMA/Albumin ratio and serum Bilirubin [Table/Fig-5]. The r-value was 0.4051and p-value was 0.0116.
Graph showing positive correlation between MELD score and IMA/Albumin using correlation coefficient test.
Graph showing positive correlation between Bilirubin and IMA/Albumin using correlation coefficient test.
A positive correlation was also obtained between IMA/Albumin ratio and ALP [Table/Fig-6] with r-value of 0.4329 and p-value of 0.0066.
Graph showing positive correlation between ALP and IMA/Albumin using correlation coefficient test.

There was no correlation between IMA or IMA/Albumin ratio and AST, ALT, GGT and PT INR.
Discussion
The current study shows significant increase in serum IMA levels and IMA/Albumin ratio in CLD patients compared to apparently healthy subjects. Studies have shown that in several conditions, IMA is elevated along with other parameters of oxidative stress like total oxidant status levels and oxidative stress index [18]. This indicates that similar molecular event occurs in CLD and that oxidative stress and free-radical mediated damage may be key events in the development of liver failure [19]. Cakir et al., studied IMA levels and IMA/Albumin ratio in pediatric patients with CLD and correlated the levels with Child-Pugh criteria. They showed that IMA is elevated and IMA/Albumin ratio correlated with disease severity and liver fibrosis [20].
Correlation test demonstrated a negative correlation between IMA and Albumin. This denotes that in patients with CLD, as the albumin level decreases, the level of IMA will increase. This suggests that in chronic liver failure there is qualitative alteration of albumin to IMA in addition to the quantitative reduction in albumin that occurs due to hepatocyte damage. However, the molecular events resulting in the structural alteration causing serum albumin reduction is still unknown. This qualitative alteration may explain why albumin infusion is controversial and often deemed less beneficial in the management of cirrhosis and other end stage liver diseases [21].
Human albumin being abundant in the circulation is easily targeted by several agents and other structurally modified albumin forms like peroxynitrite modified serum albumin have been recognized [22]. However from the observation in our study that albumin and IMA shows an inverse correlation, we can come to a conclusion that, although there may be other unrecognized forms of albumin, it is the IMA change that occurs in significant proportions to play a role in pathophysiological mechanisms. This also signifies that IMA must be the focus in further studies involving modified albumin forms.
Albumin is an important structural molecule produced in the liver and is intricately associated with the pathophysiological mechanisms in various forms of CLD [23]. Alterations to the structure of albumin have been observed in diseases like acute coronary syndromes, skeletal muscle ischemia and liver disease [5, 24, 25]. It has been hypothesized that fatty acids influence binding of cobalt to albumin which in turn provide an insight on the origin of IMA [26]. But an important question needing an answer is on whether the structural changes leading to the formation of molecules like IMA occur in the peripheral circulation triggered by the insult and factors released due to end organ ischemic damage or whether it is the liver that produces the modified protein in response to oxidative stress. Another question pertains to whether the current methods used to quantify albumin measures only the structurally normal protein or is inclusive of the altered forms. If future studies concentrate on the above mentioned aspects, more clarity can be attained with regard to the molecular changes occurring to albumin.
The Model for End stage Liver Disease (MELD) score was introduced in 2000 by Malinchoc et al., as a model to predict mortality in patients undergoing portosystemic shunts [12]. Three variables had an independent and significant impact on short-term, 3-month survival in patients with CLD. These variables included serum bilirubin, creatinine, the international normalized ratio (INR). Based on the MELD score, the 3 month mortality of patients with CLD is calculated. A score of 40 or more means 71.3% mortality, 30–39 denotes 52.6% mortality, 20–29 means 19.6% mortality, 10–19 denotes 6.0% mortality and <9 means 1.9% mortality [13]. Our findings show that IMA/Albumin ratio correlates with MELD score. IMA as a lone biomarker if estimated in CLD can thus indicate the 3 month mortality rate and can be used to prognosticate patients.
IMA/Albumin ratio positively correlates with bilirubin and ALP. These are elevated in CLD as a consequence of hepatic dysfunction [14]. But IMA does not correlate with other parameters and ALT which is more specific for the liver. This shows that IMA may not be specific to a pathology confined to the liver, but by determining IMA and IMA/Albumin ratio, a fair idea regarding the severity of the disease and the prognosis of the patients can be obtained. The limited sample size of this study may be a reason for these correlation findings and hence further studies needs to be done in more controlled conditions and a larger sample size.
Whether a difference in etiology can cause variations in the level of the parameters determined was not considered in the study and must be analysed in future research. Other parameters were done only on the test group patients as they were established markers used in routine clinical practice and comparison with control group was not needed. This has been mentioned in methodology. Budgetary constraint was another reason for not doing the parameters in the control group. Another limitation of the study is the lack of follow up data of the patients.
Conclusion
In conclusion it is seen that elevated serum IMA levels indicates oxidative stress and ischemia, which are prime factors in the causation and development of CLD. Decreased serum albumin in correlating with increase in IMA in CLD could indicate a qualitative change in the structure of albumin and not merely a quantitative reduction that is responsible for the loss of its physiological function which changes the basic understanding regarding the processes that cause a reduced albumin functioning in liver diseases. IMA can serve a biomarker that can be easily quantified to assess the disease severity and prognosis of CLD patients.