Evaluation of Long Term Effect of RV Apical Pacing on Global LV Function by Echocardiography
Narayan Chandra Sarkar1, Mahendra Tilkar2, Siddhant Jain3, Subrata Mondal4, Piyabi Sarkar5, Nitin Modi6
1 Associate Professor, Department of Cardiology, Sri Aurobindo Medical College and P.G Institute, Indore, MP, India.
2 Assistant Professor, Department of Medicine, Sri Aurobindo Medical College and P.G Institute, Indore, MP, India.
3 Associate Professor, Department of Cardiology, Sri Aurobindo Medical College and P.G Institute, Indore, M.P, India.
4 Professor, Department of Cardiology, L.N Medical College, Bhopal, M.P, India.
5 P.G Resident, Department of Pathology, Institute of Post Graduate Medical Education and Research and S.S.K.M Hospital, Kolkata, (W.B), India.
6 Interventional Cardiologist, CHL Apollo Hospital, Indore (M.P), India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Narayan Chandra Sarkar, Saims Campus, Flat No 402, Akansha Block, Near Indore Ujjain Highway-453555, Indore M.P., India.
E-mail: Sarkar.narayanchandra9@gmail.com
Introduction
We very often face pacemaker implanted patients during follow-up with shortness of breath and effort intolerance inspite of normal clinical parameters.
Aim
The aim of our study is to evaluate the cause of effort intolerance and probable cause of sub-clinical Congestive Cardiac Failure (CCF) in a case of long term Right Ventricular (RV) apical pacing on global Left Ventricular (LV) function non- invasively by echocardiography.
Materials and Methods
We studied 54 patients (Male 42, Female 12) of complete heart block (CHB) with RV apical pacing (40 VVI and 14 DCP). Mean duration of pacing was 58+4 months. All patients underwent 24 hours Holter monitoring to determine the percentage of ventricular pacing beats. 2-D Echocardiography was done to assess the regional wall motion of abnormality and global LV ejection fraction by modified Simpson’s rule. These methods were coupled with the Doppler derived Myocardial Performance Index (MPI), tissue Doppler imaging, and mechanical regional dyssynchrony with 3-D Echocardiography. Data were analysed from 54 RV- apical paced patients and compared with age and body surface area of 60 controlled subjects (Male 46, Female 14).
Results
Evaluation of LV function in 54 patients demonstrated regional wall motion abnormality and Doppler study revealed both LV systolic and diastolic dysfunction compare with control subjects (regional wall motion abnormality 80±6% vs 30±3% with p-value<0.0001) which is proportional to the percentage of ventricular pacing beats (mean paced beat 78%). Global LVEF 50±4% vs 60±2% (p-valve <0.0001) and MPI 0.46 ±0.12 v/s 0.36±0.09 (p-value <0.0001).
Conclusion
RV–apical pacing induces iatrogenic electrical dyssynchrony which leads to remodeling of LV and produces mechanical dyssynchrony which is responsible for LV dysfunction. Alternate site of RV pacing and/or biventricular pacing should be done to maintain biventricular electrical synchrony which will preserve the LV function.
Holter monitoring, LVEF, Wall motion abnormality
Introduction
Syncope is a common presentation of complete heart block (CHB). Syncope is defined as a transient, self-limited loss of consciousness with inability to maintain postural tone and followed by spontaneous recovery due to failure of cardiac impulse generation and /or propagation [1]. Patient may present with convulsive seizures that resemble an epileptic attack or the patient may fall without significant warning causing injuries or even death. Furman S et al., illustrated that transvenous endocardial cardiac pacing with a pacemaker electrode placed in the right ventricle (RV) apex which corrected the electrical disturbance and was a life saving technique [1].
The advent of transvenous cardiac pacing became life saving device for the management of life-threatening symptomatic heart block which resets the beating of the heart and maintain cardiac output for proper functioning of the vital organs of the body. RV apex is the preferred site for transvenous cardiac pacing from the beginning because it is easy for placement of lead which assure stability, reliability and very much convenient because of the design of the lead [2,3].
Permanent cardiac pacing is the rapidly advancing technology in cardiology in symptomatic heart block patients. Pulse generator size is reduced and low threshold drug eluding securing leads have been evolved but traditionally leads had been fixed to the RV apex. Placement of pacing lead in RV apex produces iatrogenic Left Bundle Branch Block (LBBB) which produces an abnormal late activation of the lateral wall of the LV. This electrical dyssynchrony induces differential muscle strain and fibre shortening which leads to increased work load and oxygen demand. This mechanical dyssynchrony alter cardiac haemodynamics and produces ventricular remodeling due to neuro-hormonal and electrophysiological changes. This electrical and mechanical dyssynchrony leads to both systolic and diastolic dysfunction of LV [4–6].
Short term RV pacing showed reversible abnormality of the LV function in various studies [7–9]. Cellular and subcellular abnormality has been detected in long-term cardiac pacing, but chronic ventricular mechanical dyssynchrony leading to irreversible LV dysfunction is not fully evaluated non-invasively [10,11].
Aim
The aim of our study was to evaluate the LV function after long- term RV pacing by echocardiography in our Indian setup.
Materials and Methods
This case control study was undertaken to evaluate the LV function of 54 CHB patients (Male 42, Female 12) who were paced from the RV apex aged 51-65 years (mean years age 58+7) with mean body surface area (BSA) of 1.75+.4 m2 from January 2013 to July 2015 at Sri Aurobindo Institute of Medical Sciences and P.G Institute Indore, M.P, India. Informed consent was obtained from every patient prior to the conducting of the study. Ethical clearance was obtained from Institutional ethical committee.
Inclusion Criteria
Non diabetic, non hypertensive, no history suggestive of coronary heart disease, normal LV function before pacemaker implantation as well as normal LV morphology. All valves were structurally and functionally normal and no intracardiac shunt. Studied subjects were compared with control group comprising 60 healthy subject matched with age and body surface area. All control subjects were asymptomatic, non-diabetic, non-hypertensive, no cardiovascular illness and normal resting ECG.
Exclusion Criteria
Known diabetic, hypertensive, coronary heart disease (CHD), Chronic Obstructive Pulmonary Disease (COPD) and other co-morbid diseased patients were excluded from the studied group.
Holter Monitoring
All the paced patients underwent 24 hours holter monitoring by Philips Holter Machine 1810 with Zymed Algorithm to evaluate the pacemaker dependency.
Echocardiography
Transthoracic Echocardiography was performed by Philips IE-33 to evaluate the LV function of the patients. Echocardiography was performed with the same machine and same person in both paced and control group. Mitral inflow velocity and tissue Doppler imaging was done as per recommendation of the American Society of Echocardiography. Pulse Doppler echocardiography was performed to determine the timing of cardiac events by recording mitral inflow and aortic out flow pattern. 2-D Echocardiographic images of the LV in the flour, three and two chamber views were taken. Parasternal short axis view at the basal mid and apical level was acquired to see the regional wall motion abnormality. LV ejection fraction (LVEF) was measured using biplane method according to modified Simpson’s rule.
Mechanical Dyssynchrony
Septal to posterior wall activation time is determined by m-mode echocardiography. Interval ≥130 m sec was taken as marker of dyssynchrony. Doppler tissue imaging in the proximal septum and lateral wall is determined and delay > 30 m. sec was taken as significant. Mechanical dyssynchrony was corroborated by 3-D echocardiography using in novel speckle tracking system and longitudinal, circumferential and radial strains were quantified in a 17 segments modal.
Full volume real time 3-D echocardiogram recorded from apical view with 4 chamber, 2 chamber and apical long axis view have been extracted from a full volume single cycle with Doppler tissue imaging to determine the time to peak velocity in multiple regions.
Myocardial Performance Index (MPI)
M.P.I is the universally accepted non invasive Doppler flow derived intervals which assess both systolic and diastolic LV function. It is defined as a ratio of the sum of Isovolumetric Contraction Time (IVCT) and Isovolumetric Relaxation Time (IVRT) to ejection time. MPI is deducted by substracting ejection time (ET) from Total Systolic Time (TST) and divided by Ejection Time (ET) [Table/Fig-1].

Or, Isovolumetric Contraction Time (IVCT) is added with Isovolumetric Relaxation Time (IVRT) which is divided by ejection time.
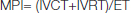
(Normal value of MPI is ≤ 0.40.)
Calculation of Myocardial Performance Index(MPI)
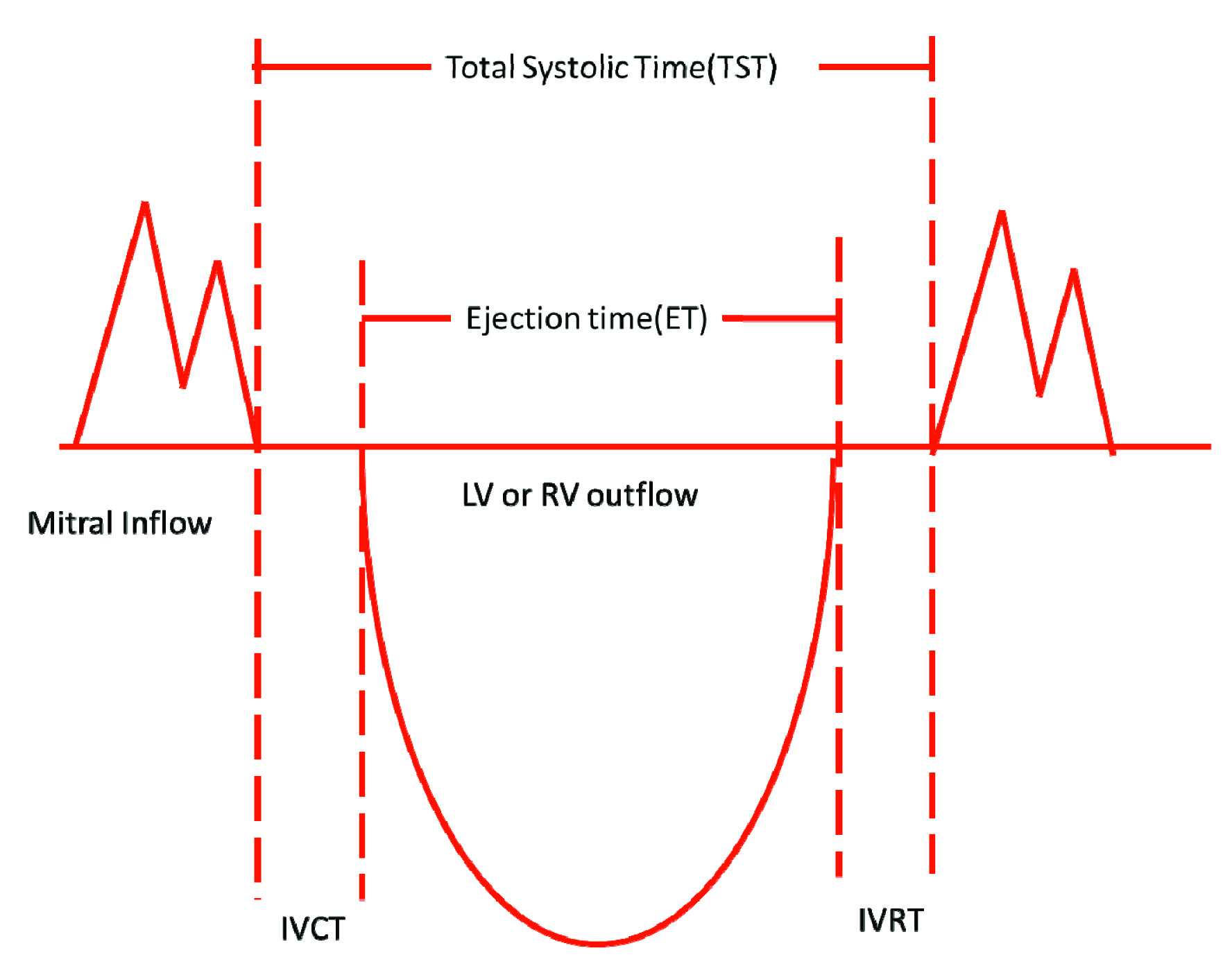
IVCT is the time period between closing of mitral valve and opening of aortic valve with doppler study by spectral doppler signal. The sample volume is kept between mitral inflow and LV or RV outflow tract. IVRT is the time interval between closing of LV or RV outflow tract and opening signal of next mitral inflow. The sample volume is kept between LV or RV outflow and mitral inflow tract.
Assessment of LV Diastolic Function
Mitral inflow by CW: IVRT, E-wave and A-wave filling velocity, E/A ratio, E-wave deceleration time are determined. Tissue Doppler mitral annular velocity: From four-chamber view, the sample volume in positioned on the annulus near the insertion site of mitral valve at the septal (medial) and lateral site with PW and e’ and a’ are visualized and ascertained.
Statistical Analysis
Statistical analysis was done using GraphPad (Demo version). A p-value was set at <0.05 as statistically significant. Student t-test was applied to see the significant difference in mean of quantitative variables in two groups
Results
We studied 54 patients prospectively (male 42, female 12) [Table/Fig-2] of complete heart block with RV apical pacing patients (VVI-40, DCP-14). Mean duration of pacing was 58±4 months. All the paced patients underwent 24 hours holter monitoring to evaluate the pacemaker dependency. 50 patients out of 54 paced patients were 100% pacemaker dependant as revealed by holter monitoring. The paced QRS interval ranged from 120-180 msec with mean QRS axis -45°. Echocardiography shows hypokinesia at the RV apex, anterior, infero- lateral wall and dyskinesia in the LV apex. These regional wall motion abnormality when compared with control subject revealed 80±6% vs. 30±3% (p-value<0.0001). Doppler study revealed both systolic and diastolic dysfunction in the paced group compared with the control group as manifested by LVEF 50±4% vs 60±2%(p-value<0.0001) which is proportional to the percentage of ventricular pacing beats (mean pace beats 78%), MPI was 0.46±0.12 in the paced subject vs 0.36±0.09 in the control group (p-value<0.0001) [Table/Fig-3].
Distribution of study subjects.
| Gender | Case | Control |
|---|
| Male | 42 | 46 |
| Female | 12 | 14 |
| Total | 54 | 60 |
Evaluation of LV function
| Evaluation of LV Function | Case (54) | Control (60) | p-Value |
|---|
| LVEF | 50±4% | 60±2% | <0.0001** |
| Wall Motion Abnormality | 80±6% | 30±3% | <0.0001** |
| MPI | 0.46±0.12 | 0.36±0.09 | <0.0001** |
**p-value<0.05 significant
Discussion
There are positive evidence of myocardial perfusion and functional abnormalities in the Left Bundle Branch Block (LBBB). Various studies showed myocardial perfusion defect in LV which has been evaluated by exercise myocardial scintigraphy without any Coronary Artery Disease (CAD) [12–14]. Various clinical and experimental studies showed regional blood flow reduction in LBBB without any obvious CAD. Asynchronous septal activation produces electrical dyssynchrony which might be the cause of asymmetric LV wall thickness and ultimately leads to wall motion abnormality and LV dysfunction [15].
Animal Experimental Studies
Various investigators placed the pacing lead in the RV apex in their animal model and thus produced iatrogenic LBBB which leaded to asynchronous electrical activation leading to morphological dyssynchrony of LV [16–18]. They showed alteration of myocardial blood flow and impairment of LV function in their studied animal model [19,20]. Morphological and histological changes demonstrated due to this redistribution of myocardial blood flow and ultimately impairment of LV function [21,22]. Prinzen et al., showed loss of contractile function in early activated region and compensatory hypercontractility in the late activated region by MRI [3].
Skalidi’s et al., showed thinning of myocardium at the pacing site due to asynchronous ventricular activation which was detected in animal models [23]. When the pacing wire was put on the basal region of the animal model there was no impairment of global LV function. RV apical pacing results inhomogeneous ventricular activation due to repaid and slow impulse propagation. Pacing at the LV basal region showed consistent propagation of activation [24]. Result of difference in myocardial activation may be the causative factor of larger hypo-functioning area of the RV apical pacing. In contrast of RVOT (Right Ventricular Outflow Tract) pacing which maintained synchronous ventricular activation and preserved myocardial function and thus prevent the development of myofibrillar disarray [17].
Human Studies
Several human studies have demonstrated that RV apical pacing which altered regional myocardial blood flow and adrenergic innervations due to asynchronous ventricular activation ultimately impaired LV systolic and diastolic function with normal coronary arteries [25,26]. Tse et al., proved in his animal model study that degree of regional perfusion defect and wall motion abnormality depend on duration of RV pacing and ultimately deteriorates LV function, the more the duration of pacing the more the LV dysfunction [27].
To prevent those long term adverse effects of RV apical pacing, alternate permanent pacing site had been investigated. RVOT and RV septal site were taken as alternate site of permanent pacing with screwing lead for long term safety and achieving normal contraction pattern as this particular site of the ventricle depolarizes first. The effect of RVOT pacing revealed mixed results. Schwaab et al., revealed significant early haemodynamic benefit of early RVOT pacing in comparison of RV apical pacing [28]. But Victor et al., did not find any symptomatic and haemodynamic benefit after 3 months of follow up [29].
Tse et al., did not notice any improvement of LV function during follow-up period for 6 month in their comparative studies of alternate site of pacing but noticed significant improvement in global LV systolic and diastolic function after 18 months [30]. They concluded that in both RVOT and apical pacing site there was appreciable regional wall motion abnormality which could not be compensated by increasing wall motion in the remote site during the early 6 month period. So there was no appreciable improvement of LV function in their comparative study during early 6 month. This might be the cause of failure to show the benefit of RVOT pacing over RV apical pacing during follow up for 6 months. The other various studies which were followed up for more than 12-18 months showed improved LV function of RVOT pacing compared to RV apical pacing [31,32].
Biventricular Pacing
In this mode of pacing both ventricles are simultaneously activated. One pacing lead is put in the RV apex to activate RV and another lead is transvenously placed through a branch of coronary sinus on the postero-lateral wall of LV which activates LV. This biventricular lead placement maintains the physiological synchronous activation of the heart which prevents morphological remodelling of the ventricle and helps to maintain normal LV function [33].
Limitations
The limitation of the current study is small sample size. A large scale cohort study with radio nuclide myocardial perfusion assessment of the LV may be done for correlation of the non invasive assessment of the LV function by echocardiography in the subject of conventional RV apical pacing for prolonged period.
Conclusion
Complete heart block patient with permanent pace maker pacing lead in the RV apex dyssynchronises the electrical activation and produces morphological remodelling of the left ventricle which leads to progressive worsening of global LV function over long time. Alternate site of pacing at RVOT and /or biventricular pacing which maintain synchronous ventricular activation and preserves both global LV systolic and diastolic function over long term cardiac pacing.
**p-value<0.05 significant
[1]. Furman S, Schwedel J, An intracardiac pacemaker for Stokes-Adams seizuresN Eng J Med 1959 261:943-48. [Google Scholar]
[2]. Bedotto JB, Grayburn PA, Black WH, Alterations in LV relaxation during atrioventricular pacing in humansJ Am Coll Cardiol 1990 15:658-64. [Google Scholar]
[3]. Prinzen FW, Hunter WC, Wyman BT, McVeigh ER, Mapping of regional myocardial strain and work during ventricular pacing: experimental study using magnetic resonance imaging taggingJ Am Coll Cardiol 1999 33:1735-42. [Google Scholar]
[4]. Prinzen FW, Augustijn CH, Arts T, Allessie MA, Reneman RS, Redistribution of myocardial fiber strain and blood flow by asynchronous activationAm J Physiol 1990 259:H300-H8. [Google Scholar]
[5]. Delhaas T, Arts T, Prinzen FW, Reneman RS, Regional fibre stress-fibre strain area as an estimate of regional blood flow and oxygen demand in the canine heartJ Physiol 1994 477:481-96. [Google Scholar]
[6]. Giudici MC, Thornburg GA, Buck DL, Coyne EP, Walton MC, Paul DL, Comparison of RV outflow tract and apical lead permanent pacing on cardiac outputAm J Cardiol 1997 79:209-12. [Google Scholar]
[7]. Zile MR, Blaustein AS, Shimizu G, Gaasch WH, RV pacing reduces the rate of LV relaxation and fillingJ Am Coll Cardiol 1987 10:702-09. [Google Scholar]
[8]. Karpawich PP, Rabah R, Haas JE, Altered cardiac histology following apical RV pacing in patients with congenital atrioventricular blockPacing Clin Electrophysiol 1999 22:1372-77. [Google Scholar]
[9]. Adomian GE, Beazell J, Myofibrillar disarray produced in normal hearts by chronic electrical pacingAm Heart J 1986 112:79-83. [Google Scholar]
[10]. Aryl. Goldberger, Zacharcy D, Goldberger Alexei shvilkin, Goldberger’s Clinical Electrocardiography 2013 8th editionElsevier:2-4. [Google Scholar]
[11]. Hirzel HO, Senn M, Nuesch K, Thallium-201 scintigraphy in complete left bundle branch blockAm J Cardiol 1984 53:764-69. [Google Scholar]
[12]. Grines CL, Bashore TM, Boudoloulas H, Olson S, Shafer P, Wooley CF, Functional abnormalities in isolated left bundle branch block: the effect of interventricular asynchronyCirculation 1989 79:845-53. [Google Scholar]
[13]. Karpawich PP, Justice CD, Chang CH, Septal ventricular pacing in the immature canine heart: a new perspectiveAm Heart J 1991 121:827-33. [Google Scholar]
[14]. Fu L, Imai K, Okabe A, Mashima S, Takahashi N, Kato K, A possible mechanism for pacemaker-induced T-wave changesEur Heart J 1992 13:1173-79. [Google Scholar]
[15]. Matthijs FM, van Oosterhout, Frits WP, Theo A, Robert SR, Chronic ventricular pacing at physiological heart rate affects local myocardial blood flow and local wall thickness [abstract]J Am Coll Cardiol 1996 27(Suppl A):344A [Google Scholar]
[16]. Waldman LK, Covell JW, Effects of ventricular pacing on finite deformation in canine left ventriclesAm J Physiol 1987 252:H1023-30. [Google Scholar]
[17]. Karpawich PP, Justice CD, Cavitt DL, Chang CH, Developmental sequelae of fixed-rate ventricular pacing in the immature canine heart. An electrophysiologic, haemodynamic, and histopathologic evaluationAm Heart J 1990 119:1077-83. [Google Scholar]
[18]. Buckingham TA, Candinas R, Attenhofer C, Systolic and diastolic function with alternate and combined site pacing in the right ventriclePacing Clin Electrophysiol 1998 21:1077-84. [Google Scholar]
[19]. Rosenqvist M, Bergfeldt L, Haga Y, Ryden J, Ryden L, Owall A, The effect of ventricular activation sequence on cardiac performance during pacingPacing Clin Electrophysiol 1996 19:1279-86. [Google Scholar]
[20]. Prinzen FW, Cheriex EC, Delhaas T, Asymmetric thickness of the LV wall resulting from asynchronous electric activation. a study in dogs with ventricular pacing and in patients with left bundle branch blockAm Heart J 1995 130:1045-53. [Google Scholar]
[21]. Lee MA, Dae MW, Langberg JJ, Effects of long-term RV apical pacing on LV perfusion, innervation, function and histologyJ Am Coll Cardiol 1994 24:225-32. [Google Scholar]
[22]. Van Oosterhout MF, Prinzen FW, Arts T, Asynchronous electrical activation induces asymmetrical hypertrophy of the LV wallCirculation 1998 98:588-95. [Google Scholar]
[23]. Skalidis EI, Kochiadakis GE, Koukouraki SI, Myocardial perfusion in patients with permanent ventricular pacing and normal coronary arteriesJ Am Coll Cardiol 2001 37:124-29. [Google Scholar]
[24]. Wyman BT, Hunter WC, Prinzen FW, McVeigh ER, Mapping propagation of mechanical activation in the paced heart with MRI taggingAm J Physiol 1999 276:881-91. [Google Scholar]
[25]. Rosenqvist M, Isaaz K, Botvinick EH, Relative importance of activation sequence compared to atrioventricular synchrony in LV functionAm J Cardiol 1991 67:148-56. [Google Scholar]
[26]. Nielsen JC, Bottcher M, Nielsen TT, Pedersen AK, Andersen HR, Regional myocardial blood flow in patients with sick sinus syndrome randomized to long-term single chamber atrial or dual chamber pacing—effect of pacing mode and rateJ Am Coll Cardiol 2000 35:1453-61. [Google Scholar]
[27]. Tse HF, Lau CP, Long-term effect of RV pacing on myocardial perfusion and functionJ Am Coll Cardiol 1997 29:744-49. [Google Scholar]
[28]. Schwaab B, Frohlig G, Alexander C, Influence of RV stimulation site on LV function in atrial synchronous ventricular pacingJ Am Coll Cardiol 1999 33:317-23. [Google Scholar]
[29]. Victor F, Leclercq C, Mabo P, Optimal RV pacing site in chronically implanted patients. a prospective randomized crossover comparison of apical and outflow tract pacingJ Am Coll Cardiol 1999 33:311-16. [Google Scholar]
[30]. Tse HF, Yu C, Wong KK, Tsang V, Leung YL, Ho WY, Functional abnormalities in patients with permanent RV pacing. The effect of sites of electrical stimulationJ Am Coll Cardiol 2002 40:1451-48. [Google Scholar]
[31]. Flevari P, Leftheriotis D, Fountoulaki K, Panou F, Rigopoulos AG, Paraskevaidis I, Long-term non outflow septal versus apical RV pacing: relation to LV dyssynchronyPacing Clin Electrophysiol 2009 32:354-62. [Google Scholar]
[32]. Vanerio G, Vidal JL, Banizl PF, Aguerre DB, Vlana P, Tejada J, Medium- and longterm survival after pacemaker implant: improved survival with RV outflow tract pacingJ Int Cardiac Electrophysiol 2008 21:195-201. [Google Scholar]
[33]. Aryl. Goldberger, Zacharcy D, Goldberger Alexei shvilkin, Goldberger’s Clinical Electrocardiography 2013 8th editionElsevier:204-5. [Google Scholar]