Heart Rate Changes in Electroacupuncture Treated Polycystic Ovary in Rats
Mukilan Ramadoss1, Gunasekaran Ramanathan2, Angelie Jessica Subbiah3, Chidambaranathan Natrajan4
1 Senior Lecturer, Department of Physiology, CSI College of Dental Sciences and Research, Madurai, India.
2 Professor, Department of Physiology, VMKV Medical College, Salem, India.
3 Senior lecturer, Department of Anatomy, CSI College of Dental Sciences and Research, Madurai, India.
4 Professor, Department of Pharmacology, KM College of Pharmacy, Madurai, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Gunasekaran Ramanathan, Professor, Department of Physiology, VMKV Medical College, Salem- 636308, India.
E-mail: gunasekaranrr@gmail.com
Introduction
Polycystic Ovary Syndrome (PCOS) is a common metabolic disorder, it affects both humans and animals. It may induce coronary heart disease, obesity and hyperandrogenism. Previous studies show that Low frequency Electroacupuncture (EA) have an effect on PCOS, however the exact pathway is unclear.
Aim
To find the effect of EA on autonomic activity of the heart in Estradiol Valerate (EV) induced PCOS rats.
Materials and Methods
Heart rate variability (HRV) was assessed in 3 groups: 1) Control; 2) PCOS rats; and 3) PCOS rats after EA treatment (n=8 in each group). From the time domain analysis and frequency domain analysis (linear measures) HRV analysis was done. EA stimulation was given at low frequency of 2Hz for 15 min on alternate days for 4-5 weeks. Collected data were statistically analysed using One-Way Analysis of Variance with the application of multiple comparisons of Tukey test.
Results
EA treatment group shows significant reduction in Heart Rate (HR) and low frequency, high frequency ratio (LF/HF); and increase in RR interval, Total Power (TP) when compared to PCOS group.
Conclusion
The study concludes that EA treatment has a significant effect on reducing sympathetic tone and decreasing HR in PCOS.
Heart rate variablity, Estradiol valerate, Polycystic ovary syndrome
Introduction
Cardiovascular disease (CVD) in one of the most common metabolic disorder in women with polycystic ovary syndrome, it is also associated with insulin resistance, hyperandrogenism and obesity [1,2]. Young women with PCOS have increased sympathetic and decreased parasympathetic activity in heart measured by heart rate variability (HRV) [3].
Autonomic imbalance in heart is related to CVD, group of women with PCOS has high risk of CVD [4]. Heart rate fluctuation is an indication of autonomic nervous system dysfunction in response to anxiety and environmental stimuli [5].
Earlier studies show EV induced PCOS animal model shows acyclicity in the ovary [6]. The induced PCOS rats have shown alterations in basal luteinizing hormone, follicle stimulating hormone and gonadotrophin releasing hormone and sympathetic activity of the heart [7]. The PCOS in rats and the human shows similar endocrinological and morphological features [8].
A serious alteration of HRV is associated with CVD [9] Alteration of HRV in different stages of the menstrual cycle was reported [10]. HRV is a non-invasive index for studying the autonomic activity of heart. The linear method of HRV is analysed by time and frequency domain. The time domain analysis measures changes in heart rate over time. The time domain parameters are driven mainly via parasympathetic innervations of heart. In short term recordings the frequency domain analysis of heart rate explains three bands very low frequency (VLF), low frequency (LF) and high frequency (HF) [11]. An alternate approach of recording ECG in conscious state allows a long term HRV study in a rat model [12]. HRV has been studied for alterations in the autonomic activity of heart during acupressure stimulation in Dysmenorrhoea [13].
The standard pharmacological treatment for PCOS is effective, but it has side effect such as super ovulation are common. Laparoscopic ovarian drilling may help in regulating ovulation in women with PCOS [14]. Manual stimulation of acupuncture needles and metformin therapy, both showed similar improvement in the endocrine and metabolic function in obese PCOS women [15]. Regular ovulations were induced in more than one third women without any side effects [16].
Earlier studies were limited in explaining the association between the PCOS and metabolic disorders.
Aim
Therefore, this study may contribute to understanding the autonomic alteration of heart in PCOS and the effect of EA on PCOS.
Materials and Methods
Animals: The study was conducted at the KM college of Pharmacy, Madurai, Tamil Nadu. The time period of the study was 3 months. 24 adult virgin cyclic rats weighing 200 -250g were housed in cages at a controlled temperature, for a period of one week 12-12 hours a day, night cycle is maintained and has free access to food and ad libitum water. The study was conducted after getting the institutional animal ethical committee approval.
PCO induction: After acclimatization the rats were divided into three groups, control (n=8), PCOS (n=8) and PCOS + EA (n=8). Each rat in the PCOS group was injected with single I.m injection of 4.5 mg of EV (Progynon deport, German Remedies). The animals were handled according to CPCSEA guidelines [17].
Vaginal smear: In all the control and experimental group rats vaginal smear was taken periodically. The EV induced PCOS rats shows prolonged diestrus stage, when compared to control that we consider it as PCOS group. The vaginal smear was the conformance test for PCOS appearance. The smears were stained using crystal violet and viewed under microscope and the predominant cell type in vaginal smear in the PCOS is leukocytes the main cell type in diestrus stage [18].
Animal preparation and HRV recording: For adaptation the animals were conditioned inside a transparent restrainer on everyday 20 minutes for a week prior to recording of HRV. The ventral surface of the animals were shaved. A conductive gel was applied over the self adhesive platinum electrode with care being taken to avoid the establishment of gel bridge between them.
The electrodes were connected to cable to reach the data acquisition system. The animal was placed inside the transparent retainer which has holes for ventilation. After 10 minutes inside the retainer, the HRV was recorded for 5 minutes in all the groups. All the recording was done at the same time between 9am to 11am under thermonueral condition. To avoid the potential acute effect of EA, no treatment was performed 24 hours before recording HRV in PCOS EA group.
HRV analysis: In time domain analysis the following index was obtained mean RR interval, mean heart rate HR, and SDNN (Standard deviation of RR interval), for frequency domain analysis, there were two major spectral component is Low frequency (LF 0.2 – 0.8 Hz) and High frequency (HF 0.8 -2.5 Hz).
Electroacupuncture treatment: Low frequency EA treatment was carried out in the PCOS group, each treatment lasted for 20 minutes for one in two days upto 4-5wks. The rats were anesthetized and suspended in harness. The needles were inserted bilaterally in the biceps femoris and erector spinae muscle in somatic segment corresponding to the innervations of the ovaries. The needles were inserted to the depth of 0.3 -0.5 cm and then connected to an electrical stimulator with the low frequency of 2Hz and the burst frequency of 80 Hz. The intensity was adjusted until the local muscle contraction valid from 0.8 – 1.3 mA. The animals in the PCOS and control groups were anesthetized and suspended in a harness and handled as a PCOS EA group and they are exempted from EA stimulation.
Statistical Analysis
The data are expressed as mean ± SD. The time and frequency domain parameters were analysed using One-Way Analysis of Variance with the application of multiple comparisons of Tukey test. Significance of p < 0.05 was considered statistically significant.
Results
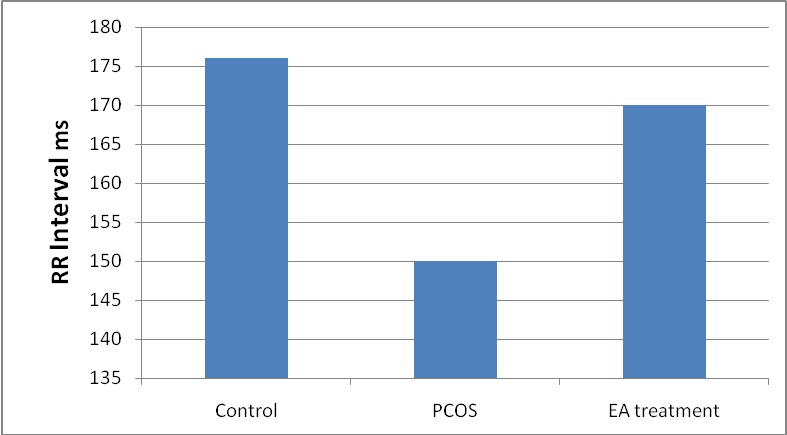
In EV induced PCOS rats there was a significant decrease in the time domain indices (mean RR, RMSSD, SDNN, and NN50) when compared to normal (p <0.05). After 4–5wks of EA treatment in EV induced PCOS rats there was a significant increase in time domain indices when compared to untreated PCOS rats (p <0.05) [Table/Fig-1,2].
Comparison of time domain analysis between PCOS, Control and EA treatment groups.
| Parameters | Control | PCOS | EA treatment |
|---|
| Mean RR | 176±3.8 | 150±3.8 * | 170.7±3.4 ** |
| SDNN | 13.6±1.4 | 10.76±1.4 * | 11.6±0.9 |
| rMSSD | 5.8±0.8 | 3.46±0.68 * | 5.5±0.8** |
Values are present in mean ± SD. Comparision with control group, * p < 0.05. and comparison with PCOS group ** p < 0.05
Mean Comparison of the RR interval in PCOS compared with control and EA treatment.
In frequency domain indices the EV induced PCOS rats there was a significant decrease in total power TP (p< 0.05) and high frequency power HF, the low frequency power LF and ratio of LF/ HF was significantly increased when compared to control. After EA treatment for 4–5wks the TP and HF was significantly increased, the LF and LF/HF ratio was significantly decreased when compared to untreated PCOS [Table/Fig-3].
Comparison of frequency domain analysis between PCOS, Control and EA treatment groups.
| Parameters | Control | PCOS | EA treatment |
|---|
| TP | 71.6±10.1 | 41.75±6.8* | 65.8±11.2** |
| LF | 15.07±1.9 | 20.32±2.6* | 16.5±1.6** |
| HF | 15.87±4.03 | 8.62±0.9* | 13.7±2.08** |
| LF/HF | 0.98±0.17 | 1.72±0.16* | 1.2±0.19** |
Values are present in mean ± SD. Comparision with control group, * P < 0.05. and comparison with PCOS group ** P < 0.05
After treatment for 4-5 weeks of EA in PCOS EA group the vaginal smear exhibited the keratinocytes, the predominant cell type during the estrus stage, indicating the reappearance of cyclicity.
Discussion
This study shows that intensive Low frequency EA given for 4 -5 weeks modulates the autonomic activity of heart by altering the HR, total power TP and LF/HF ratio in rats with EV induced PCOS. A variety of responses can be appreciated in the endocrine, metabolic and nervous system through intramuscular needle insersion and stimulation, which produces a specific pattern of afferent activity in peripheral nerve fibers [19]. In a rodent model of PCOS the acupuncture has sympathetic depression effect on the central nervous system and reduced ovarian sympathetic tone [20].
Normally variably in HR assessed by mean RR, RMSSD, and SDNN for the time domain index which indicates parasympathetic activation of heart. The overall HRV is broken down into specific frequencies provided additional information regarding the autonomic input of the heart. The oscillation of very low frequency (VLF) is due to various slow mechanisms of sympathetic activity. Low frequency (LF) is mainly by sympathetic innervations of heart. High frequency (HF) is mainly by the parasympathetic activity of heart. The LF/HF ratio measures the overall balance between sympathetic and parasympathetic system. [21]. The overall reduction in HRV represents either a reduction in LF or HF nu.
In this study, short term HRV analysis of PCOS rats showed TP was significantly reduced, which is an index of overall HRV. A significant increase in LF nu and decreased HF nu in PCOS rats showed increased sympathetic and decreased parasympathetic activity. The LF/HF ratio was increased in PCOS rats, which show the imbalance between sympathetic and parasympathetic activity. The increase in LF and LF/HF ratio represents the increased activity of the sympathetic system [22]. This study of animal model has shown the correlation with the HRV standards of human PCOS.
EV treated rats shows increased intra ovarian synthesis of nerve growth factor a strong marker of sympathetic nerve activity [23]. Increased sympathetic activity is associated with obesity [24]. A 21 year follow up study of PCOS women showed high risk of CVD [25].
Steroid induced polycystic ovary rats had higher mean systolic blood pressure (MSAP) and increased hypothalamus, pituitary adrenal axis (HPA) regulation [26]. Women with polycystic ovary had increased levels of high sensitive C- reactive protein, which is an inflammatory marker. Which may increase the risk for the development of CVD [27]. In a comparative study, women with PCOS were treated with Low frequency EA and Exercise, both have shown decreased high muscle sympathetic activity [28].
Further, repeated treatment of EA for 4 -5 weeks of PCOS rats showed the HR, RR interval returned back to normal significantly. In addition frequency domain analysis TP and LF/HF ratio was significantly raised. Overall, it indicates there is a reduction in sympathetic activity in PCOS rats.
Limitations
The limitation of this study is small sample size. This study does not include the serum analysis of sex hormones and lipid profile.
Conclusion
Polycystic ovary syndrome is a state of increased sympathetic activity which may have associated with the metabolic disorder such as CVD and reproductive disturbances. This study concludes that low frequency EA has a significant effect on PCOS rats, which reduces the HR and sympathetic activity. This non pharmacological less expensive alternative therapy can be implemented for PCOS to reduce the cardiovascular risk in patients.
Values are present in mean ± SD. Comparision with control group, * p < 0.05. and comparison with PCOS group ** p < 0.05
Values are present in mean ± SD. Comparision with control group, * P < 0.05. and comparison with PCOS group ** P < 0.05
[1]. Zakur HA, Epidemiology Clinical Manifestation and Pathophysiology of Polycystic Ovary SyndromeAdvanced studies in medicine 2003 3:s733-39. [Google Scholar]
[2]. Fagius J, Sympathetic Nerve Activity in Metabolic Control—Some Basic conceptsActa Physiol Scand 2003 177(3):337-43. [Google Scholar]
[3]. Aylin Y, Funda A, Heart Rate Variability in Young Women with Polycystic Ovary syndromeAnnals of Noninvasive Electrocardiology 2006 4:306-12. [Google Scholar]
[4]. Cussons AJ, Stuckey BG, Watts GF, Cardiovascular Disease in the Polycystic Ovary Syndrome: New Insights and PerspectivesAtherosclerosis 2006 185(2):227-39.Epub 2005 Nov 28 [Google Scholar]
[5]. Parati G, Saul JP, Di Rienzo M, Mancia G, Spectral Analysis of Blood Pressure and Heart Rate Variability in Evaluating Cardiovascular Regulation. A Critical AppraisalHypertension 1995 25(6):1276-86. [Google Scholar]
[6]. Brawer JR, Munoz M, Farookhi R, Development of the Polycystic Ovarian Condition (PCO) in the Estradiol Valerate-Treated RatBiol Reprod 1986 35(3):647-55. [Google Scholar]
[7]. Barria A, Leyton V, Ojeda SR, Lara HE, Ovarian Steroidal Response to Gonadotropins and Beta-Adrenergic Stimulation is Enhanced in Polycystic Ovary Syndrome: Role of Sympathetic InnervationEndocrinology 1993 133(6):2696-703. [Google Scholar]
[8]. Lara HE, Ferruz JL, Luza S, Bustamante DA, Borges Y, Ojeda SR, Activation of Ovarian Sympathetic Nerves in Polycystic Ovary SyndromeEndocrinology 1993 133(6):2690-95. [Google Scholar]
[9]. Kleiger RE, Miller JP, Bigger JT, Moss AJ, Decrease in Heart Rate Variability is Associated with Increased Risk of Cardiovascular DiseasesAmerican Journal of Cardiology 1987 59(4):256-62. [Google Scholar]
[10]. Tejinder KB, Singh KD, Avnish K, Effect of Different Phases of Menstrual Cycle on Heart Rate Variability (HRV)Journal of Clinical and Diagnostic Research 2015 9(10):CC01-CC04. [Google Scholar]
[11]. Fintel DJ, Martin GJ, Heart rate variability 1989. An updateJournal of Electrocardiology 1990 22(SUPPL):204-08. [Google Scholar]
[12]. Pereira JP, Moacir M, Rodrigues FP, Emiliano M, José HM, Noninvasive Method for Electrocardiogram Recording in Conscious Rats: Feasibility for Heart Rate Variability AnalysisAn. Acad. Bras. Ciênc 2010 2(82):431-37. [Google Scholar]
[13]. Archana R, Philominal V, Shyamala T, Effect of Acupressure and Changes in Heart Rate Variability in DysmenorrhoeaRecent Research in Science and Technology 2011 3(10):1-6. [Google Scholar]
[14]. Niraj K Y, Sirisha Rao G, Outcome of Ovarian Drilling in Women with Polycystic Ovary SyndromeJournal of Clinical and Diagnostic research 2015 9(2):QC01-QC03. [Google Scholar]
[15]. Lai MH, Ma HX, Yao H, Liu H, Song XH, Huang WY, Effect of Abdominal Acupuncture Therapy on the Endocrine and Metabolism in Obesity-Type Polycystic Ovarian Syndrome PatientsZhen Ci Yan Jiu 2010 35(4):298-302. [Google Scholar]
[16]. Chen BY, Acupuncture Normalizes Dysfunction of Hypothalamic-Pituitary-Ovarian AxisAcupunct Electrother Res 1997 22(2):97-108. [Google Scholar]
[17]. Committee for the purpose of control and supervision of experiments on animals (CPCSEA). Available from: http://moef.nic.in/modules/divisions/cpcsea/[Last accessed on 2016 Jan 27] [Google Scholar]
[18]. Benrick A, Maliqueo M, Miao S, Villanueva JA, Feng Y, Ohlsson C, Resveratrol is Not as Effective as Physical Exercise for Improving Reproductive and Metabolic Functions in Rats with Dihydrotestosterone-Induced Polycystic Ovary SyndromeEvid Based Complement Alternat Med 2013 Epub 2013 Apr 8 [Google Scholar]
[19]. Kagitani F, Uchida S, Hotta H, Aikawa Y, Manual Acupuncture Needle Stimulation of the Rat Hindlimb Activates Groups I, II, III and IV Single Afferent Nerve Fibres in the Dorsal Spinal RootsJpn J Physiol 2005 55(3):149-55. [Google Scholar]
[20]. Dissen GA, Garcia-Rudaz C, Paredes A, Mayer C, Mayerhofer A, Ojeda SR, Excessive Ovarian Production of Nerve Growth Factor Facilitates Development of Cystic Ovarian Morphology in Mice and is a Feature of Polycystic Ovarian Syndrome in HumansEndocrinology 2009 150(6):2906-14. [Google Scholar]
[21]. Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ, Power Spectrum Analysis of Heart Rate Fluctuation: A Quantitative Probe of Beat-to-Beat Cardiovascular ControlScience 1981 213(4504):220-22. [Google Scholar]
[22]. Task Force of the European Society of Cardiology and the North American Society of Pacing and ElectrophysiologyHeart rate variability: standards of measurement, physiological interpretation and clinical useCirculation 1996 93(5):1043-65. [Google Scholar]
[23]. Lara HE, Dissen GA, Leyton V, Paredes A, Fuenzalida H, Fiedler JL, Ojeda SR, An increased intraovarian synthesis of nerve growth factor and its low affinity receptor is a principal component of steroid-induced polycystic ovary in the ratEndocrinology 2000 141(3):1059-72. [Google Scholar]
[24]. Chintala KK, Krishna BH, N MR, Heart rate variability in overweight health care students: correlation with visceral fatJ Clin Diagn Res 2015 9(1):CC06-CC08. [Google Scholar]
[25]. Schmidt J, Landin-Wilhelmsen K, Brännström M, Dahlgren E, Cardiovascular disease and risk factors in PCOS women of postmenopausal age: a 21-year controlled follow-up studyJ Clin Endocrinol Metab 2011 96(12):3794-803. [Google Scholar]
[26]. Stener-Victorin E, Ploj K, Larsson BM, Holmäng A, Rats with steroid-induced polycystic ovaries develop hypertension and increased sympathetic nervous system activityReprod Biol Endocrinol 2005 3:44 [Google Scholar]
[27]. Boulman N, Levy Y, Leiba R, Shachar S, Linn R, Zinder O, Increased C-reactive protein levels in the polycystic ovary syndrome: a marker of cardiovascular diseaseJ Clin Endocrinol Metab 2004 89(5):2160-65. [Google Scholar]
[28]. Stener-Victorin E, Jedel E, Janson PO, Sverrisdottir YB, Low-frequency electroacupuncture and physical exercise decrease high muscle sympathetic nerve activity in polycystic ovary syndromeAm J Physiol Regul Integr Comp Physiol 2009 297(2):R387-95.doi: 10.1152/ajpregu.00197.2009. Epub 2009 Jun 3 [Google Scholar]