Hypertension has emerged as a major public health problem in developing as well as developed countries. It is very common among the population, affecting 1 in 3 adults worldwide [1] which translates to nearly one billion in absolute numbers in the year 2000 and this is expected to grow to more than 1.5 billion by 2025 [2]. Increasing prevalence of hypertension is a documented public health problem in India as it predisposes to cardiovascular diseases [3,4]. A recent study by Sachdev, among tribal population of Rajasthan has shown 16% to 30% prevalence of hypertension among different tribes [5]. In 1870, Frederick Mohamed was the first to note the association of Serum uric acid (SUA) and Essential Hypertension (EHT) [6] and a recent re-evaluation of the Framingham Heart Study data has also suggested that a higher SUA level is associated with increased risk of hypertension [7]. Further, studies have even shown that hyperuricaemia is more commonly associated with primary EHT than in secondary hypertension, at least in adolescents [8]. Traditional modifiable risk factors have been extensively evaluated but there is a pressing need to identify additional treatable risk factors that are easily measured and highly prevalent in general population. Hyperuricaemia could be such potentially modifiable & treatable risk factor, which might reduce the incidence of EHT.
Hence, the present study was designed to establish the association between the SUA and risk of development of EHT in the population of Southern Rajasthan as there is dearth of literature for Indian scenarios especially in Rajasthan.
Materials and Methods
The present study is a part of a bigger project carried out in the Department of Physiology of a tertiary care hospital. This cross-sectional, case control study included 125 subjects, chosen randomly from Medicine OPD and healthy volunteers like clinical and nonclinical staff of a hospital and individuals comings to hospital for health checkup during August 2013 to July 2014.
On the basis of a pilot study in our hospital, we calculated the incidence of new cases of hypertension to be 35%. We also found out the percentage of healthy controls, after meeting all the inclusion and exclusion criteria, to be around 22%. The sample obtained, was proportionally divided into cases and controls in the ratio of approximately 3:2.
All the age matched (20-50 years) and sex matched subjects were broadly divided into two groups (A&B); group A comprised of newly diagnosed cases of EHT (n=75) whereas group B had healthy normotensive controls (n=50). The subjects with gout, diabetes mellitus, gestational hypertension and/or secondary hypertension caused by renal disorders, metabolic disorders, fluid volume disturbances, endocrinal disorders etc. were excluded from both the groups. Smokers, alcohol consumers and patient using medication for hypertension were also excluded from both the groups.
After obtaining the ethical clearance from the institutional ethical committee, the data from both the groups were collected in a detailed proforma along with requisite physical examination. The biochemical parameters; SUA, Serum creatinine and fasting blood glucose levels, were estimated in both groups, for which 3 ml of blood was drawn after an overnight fast (12 h) by venous puncture. After clotting of blood, serum was separated by centrifugation at 3000 rpm for 10 minutes and used for biochemical analysis. The SUA level was measured on semi auto-analyser by Modified Trinder method [9]. Serum creatinine and fasting blood glucose levels, were estimated by Jaffe’s Method [10] and enzymatic method, using Glucose Oxidase (GOD) and Peroxidase [11] respectively. Serum creatinine and fasting blood glucose levels, were estimated to exclude renal disorder and diabetes mellitus respectively.
Statistical Analysis
The data was analysed by using Statistical Package for the Social Sciences (SPSS) Version 16.0. Association between EHT and hyperuricaemia was tested by Chi-square test and Odds ratio. Comparison of mean SUA level between group A and group B was tested by Student t-test.
Results
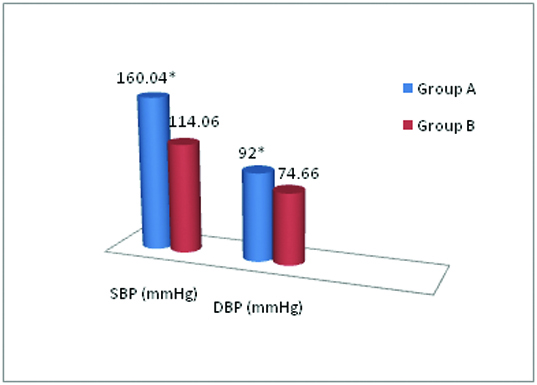
The mean age of group A and B were 40.25 ± 7.71 and 37.46 ± 8.09 years respectively (p<0.001). The mean systolic BP was 160.04 ± 16.77 mmHg and 114.06 ± 5.79 mmHg and the mean diastolic BP was 92.0 ± 10.15 mmHg and 74.66 ± 6.23 mmHg in group A and B respectively. The difference in mean systolic and diastolic BP was statistically significant (p<0.0001) between the two groups [Table/Fig-1].
Mean systolic & diastolic blood pressure in both the groups.
* p<0.0001
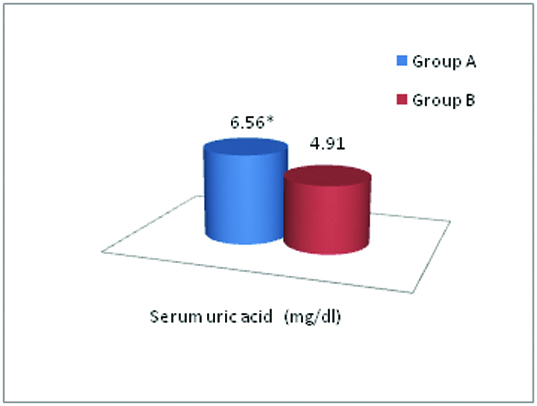
In addition, we found that the mean SUA level was 6.56 ± 0.76 mg/dl and 4.91 ± 0.97 mg/dl in group A & B respectively, which was significantly higher in group A as compared to group B (p<0.001) [Table/Fig-2]. [Table/Fig-3] shows distribution of the study subjects according to hyperuricaemia in group A and group B.
Comparison of SUA level between group A and B.
* p<0.001
Distribution of the study subjects according to hyperuricaemia in group A and group B.
| Particulars | Total no. of subject | Subject with Hyperuricaemia | Percentage | Odds Ratio | p-value |
|---|
| Group A | 75 | 28 | 37.33% | 3.66 | <0.01 |
| Group B | 50 | 7 | 14% |
Odds Ratio (OR) = 3.66, p-value <0.01, p value reached from Chi-square test
Hyperuricaemia is defined as plasma uric acid level greater than 6.8 mg/dl at physiological temperature (37°C) and neutral pH [12].
Discussion
As we know that a number of factors could affect blood pressure but they cannot completely explain the cause of EHT, which hints towards some unidentified risk factors associated with it. Since 1870, high SUA, a kind of purine metabolite, has been linked with EHT but of late it has just become diagnostic indicator of gout and renal functions, losing its significance in the pathogenesis of hypertension, cardiovascular and cerebrovascular diseases. From past centuries, a large number of epidemiological and clinical trials [7,8,13–15] have confirmed that the increased level of SUA is closely related to hypertension and other cardiovascular and cerebrovascular diseases but the lost significance of this indicator has led to surgence of the present study.
This study reflects the preclinical hyperuricaemia, as a potential risk factor for the development of EHT. An increasing trend was observed in mean serum uric acid level from control to hypertensive cases (p<0.001). These results were in concordance with several other studies which have also quoted significant increase in SUA level in essential hypertensive cases [16–19].
Further, the number of individuals with hyperuricaemia were significantly higher in group A than group B. This finding is in concurrence with other studies, which also reported 30.6% (OR= 2.13, p<0.05) and 28.8 % (OR=2.55, p<0.001) cases had hyperuricaemia with EHT respectively [16,17]. These results indicate a definite association between hyperuricaemia and EHT.
Hence, preclinical hyperuricaemia is a predictive tool for early detection of risk of development of hypertension. Several mechanisms have been suggested on the role of hyperuricaemia in the induction of systemic hypertension. Elevated SUA level lowers the endothelial nitric oxide level, by reducing nitric oxide synthase in the macula densa of the kidney with activation of renin-angiotensin system and mediates renal vasoconstriction that leads to uric acid mediated hypertension [13,20]. Persistent renal vasoconstriction may contribute to arteriosclerosis and the development of salt sensitive hypertension, even if hyperuricaemia is corrected [21]. This mechanism was demonstrated in animal studies by Mazzali et al., in which rats developed high blood pressure in about 3 to 5 weeks after raised SUA level; was induced by the administration of oxonic acid which is an inhibitor of uricase [22]. Animal and human studies have repeatedly shown an independent association of hyperuricaemia with early hypertension [8,14,19] and hypertension is a well documented preliminary risk factor for the development of coronary artery disease (CAD). In one small study, the use of allopurinol, a xanthine oxidase (XO) inhibitor, resulted in reduction of BP in adolescents with newly diagnosed hypertension with hyperuricaemia [23]. In another small study, UA lowering therapy with either allopurinol or probenecid, a uricosuric agent, significantly reduced BP in prehypertensive obese adolescent with hyperuricaemia irrespective of UA lowering mechanism [15]. Although, several international authorities such as the Joint National Committee on prevention, detection, evaluation and treatment of high blood pressure and the American Heart Association have not recognized elevated SUA as an important risk factor for CAD, but studies in humans with asymptomatic hyperuricaemia have demonstrated its association with EHT, CAD, obesity, kidney disease and metabolic syndrome [13].
The preclinical asymptomatic rise in SUA, though largely ignored, is a potential risk indicator for EHT. Hence, this study recommends the screening of SUA to detect the risk of developing EHT in future.
Limitations
First, this was a cross-sectional study and so did not permit us to make any inference on the causal relationship between uric acid and hypertension. Secondly, the limited sample size also limited the power of the analysis. A further study designed as a prospective randomized follow up study with a larger sample size would be required to substantiate the results of the present study.
Conclusion
The mean SUA levels and the frequency of hyperuricaemia were significantly higher in newly diagnosed cases of EHT as compared to normotensive healthy controls. Though the SUA level was within the normal reference range, it was significantly elevated in newly diagnosed cases of EHT. In clinical practice, measurement of SUA level may help to detect the risk of development of EHT. Moreover, further studies can be carried out to evaluate the SUA as a modifiable risk factor, to lower the incidence of EHT per se.
Odds Ratio (OR) = 3.66, p-value <0.01, p value reached from Chi-square test