Acute kidney injury (AKI) is associated with increased mortality and morbidity in undeveloped and developing countries; it is documented by acute elevation of serum creatinine, blood urea nitrogen (BUN) and occurring in hours to days to weeks [1]. The aetiology of Acute Renal Failure (ARF) is divided into pre renal, renal and post renal. Pre renal failure the most common form of ARF is mainly due to decrease in renal blood flow. Before kidney damage occurs, if the cause of the decreased renal blood flow is identified and corrected, the damage is reversible and it accounts for 40-70% of cases [2]. Intrinsic ARF involves damage to parenchymal and it can affect the blood vessels, glomeruli, tubules and/or the interstitium [3]. The most frequent cause is acute tubular necrosis, a disorder caused by ischemic or nephrotoxic injury to the kidney [4]. Other intrinsic renal causes are interstitial nephritis, atheroembolic disease [5] and glomerulonephritis and rhabdomyolysis [6]. Intrinsic renal causes accounts for 10-50% of cases [7]. Now a days rhabdomyolysis, also one of the common causes of ARF, is a condition in which damaged skeletal muscle tissue breaks down rapidly. Breakdown products of damaged muscle cells are released into the bloodstream some of these, such as the protein and myoglobin are harmful to the kidneys and may lead to kidney failure [8]. Vanholder et al., reported that 10–40% of cases with rhabdomyolsis to develop ARF [9].
Treatment and management of ARF are, stop the medications causing ARF, treating the cause, correcting the electrolyte, fluid imbalance, diuresis and renal replacement therapy (RRT). RRT includes life supporting procedures such as hemodilaysis, peritoneal dialysis, hemofiltration and renal transplant. Despite these, mortality in patients with ARF remains high and this may be due to the severity of the disease or the adverse effects of RRT.
Current research on medicinal plants is one of the most important areas of alternative drug development research globally. Demand for the use of medicinal plants is increasing nowadays in world wide. More than 221 plants had been screened for nephroprotective (acute and chronic) activity [10].
To evaluate the antioxidant and nephroprotective activities of EEAHR in glycerol induced ARF in Wistar albino rats.
Materials and Methods
Chemicals and Reagents
2-diphenyl-1-picrylhydrazyl (DPPH), Sodium nitroprusside, Griess reagent, H2O2 solution, 2,3,5 -triphenyl-1,3,4-triaza-2-azoniacyclopenta-1,4- diene chloride (TPTZ) and Vitamin-C are purchased from Sigma Aldrich, New Delhi, India.
Plant Collection and Preparation
The plant was collected from Dindivanam, Villupuram district, Tamilnadu, India and it was identified and authenticated by Professor Dr. Narasimhan, Botanist, Madras Christian College and voucher specimen number 76/2014. Roots were air dried under shade at room temperature for 30 days and milled into a coarse powder. The obtained dried powder was subjected to continuous extraction with 95% v/v ethanol in a Soxhlet apparatus. It was extracted with ethanol for 15 cycles. The extract thus obtained was concentrated to dryness in a flash evaporator under reduced pressure and controlled temperature. The extract was stored in the refrigerator and reconstituted in 1% gum acacia before administration to animals.
Animals
The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) of Chettinad Hospitals and Research Institute in its meeting dated on 24-10-13. Adult male Wistar albino rats weighing 150-250g from our breeding stock were used in this study. The animals were housed at 24±2°C with 12:12 h light and dark cycle. They had free access to food and water. The animals were acclimatized for a period of seven days before the study. The animals were used according to the CPCSEA guidelines for the use and care of experimental animals [28].
In vitro antioxidant study
The antioxidant activity of EEAHR was evaluated by using the following methods DPPH assay, NO assay, H2O2 assay and FRAP assay. Vitamin-C was used as standard. All methods were tested by using a spectrophotometer and all the percentage of inhibition was calculated by using the following formula in the all assays.
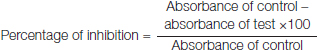
DPPH Free Radical Scavenging Assay
EEAHR was tested for free radical scavenging activity by the DPPH method of Braca et al., [29]. Free radical scavenging activity of the extract was held based on the scavenging activity of stable DPPH. EEAHR in millipore water at different concentration (25-800μg) were added to each test tube and volume was made up to 2 ml using the millipore water. To this 3 ml of 0. 004% DPPH in 95% ethanol was added and the mixtures were incubated at room temperature under dark condition for 30 min. The scavenging activity on the DPPH radical was determined by measuring the absorbance at 517 nm.
Nitric Oxide Radical Scavenging Assay:
Griess reaction reagent 3.0 ml of 10 mM sodium nitroprusside in phosphate buffer is added to 2. 0 ml of EEAHR and the reference compound in different concentrations (25-800μg). The resulting solutions were then incubated at 25°C for 60 min. The 5. 0 ml of the incubated sample and 5. 0 ml of Griess reagent (1% sulphanilamide, 0.1% naphthyethylenediaminedihydrochloride in 2% H3PO3) were added and absorbance of the chromophore formed was measured at 540nm [30].
Hydrogen Peroxide Scavenging Assay
The EEAHR was prepared at various concentrations (225-800μg) by using distilled water and mixed with 0. 6 ml of 4 mM H2O2 solution prepared in phosphate buffer (0. 1 M pH 7. 4) incubated for 10 min. The absorbance of the solution was taken at 230 nm [31].
Ferrus Reducing Antioxidant Power (FRAP) Assay
The assay mixture was held in 2. 5 ml of 300 mM acetate buffer at pH 3. 6, 0. 25 mL of 10 mM TPTZ solution in 40 mM HCl, 0. 25 mL of 20 mM FeCl3 and EEAHR at various concentrations in 0.1 ml of methanol. The absorbance was measured after 30 min incubation at 593 nm. FRAP values can be prevailed by comparing the absorption change in the test mixture with those obtained from increasing concentrations of Fe3+ and expressed as mM of Fe2+ equivalents per kg or per L of sample [32].
In Vivo Study
Induction of ARF
ARF was induced by intramuscular injection single doses of glycerol (10 ml/kg) into both the hind limbs in equal volume [33].
Experimental Design
Twenty four animals were divided into four groups of six each.
Group 1: Treated with normal saline (10 ml/kg) oral.
Group 2: Single dose of 50% glycerol (10 ml/kg) IM (Intra muscular).
Group 3: Glycerol (IM) + EEAHR 250 mg/kg oral.
Group 4: Glycerol (IM) + EEAHR 500 mg/kg oral.
The test drug EEAHR was given 60 min prior to the glycerol injection in group 3 and 4 respectively.
Assessment of Renal Failure and Sample Collection
After 24 h blood samples were collected through retro orbital sinus and urine samples by metabolic cages and animals were sacrificed. The following parameters were assessed, serum creatinine by Jaffe’s method [34], blood urea nitrogen by diacetylmonoxime method [35], total protein and albumin by biuret method [36] and urine output [37] and histopathological changes in the all groups.
Histopathology
Kidneys were washed with normal saline and then fixed in a 10% neutral buffered formalin solution, embedded in paraffin, and used for histopathological examination. Five micrometers thick sections were cut, deparaffinized, hydrated and stained with haematoxylin and eosin. The renal sections were examined for intact glomeruli, haemorrhage and capillary congestion and vacuolization of the medullary tubular cells.
Statistical Analysis
The values were expressed as mean ± SEM. The statistical analysis of data was done by One-way-analysis of variance (ANOVA) followed by Tukey’s multiple range–test used SPSS 15 version. The value of p< 0. 05 was considered to be statistically significant.
Results
In vitro Antioxidant Activity
Ethanolic extract of A. heterophyllum root in graded concentrations was tested for antioxidant activity in four different in vitro methods and compared with the standard antioxidant, vitamin C.
DPPH Assay
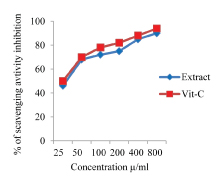
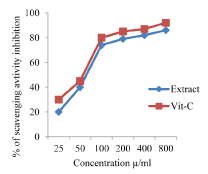
Percentage of inhibition of DPPH radicals by the EEAHR with different concentrations was 46% at 25 μg/l, 68% at 50 μg/mL and the maximum about 90% at 800 μg/mL. Standard drug, vitamin- C showed about 94% inhibition of the DPPH radicals at 800 μg/mL [Table/Fig-1].
NO Assay
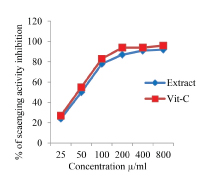
In the NO radical scavenging method, the percentage inhibition of EEAHR radicals by EEAHR was about 24% at 25μg/mL and maximum about 92% at 800 μg/mL. Vitamin-C at 800μg showed about 96% inhibitions [Table/Fig-2].
H2O2 Assay
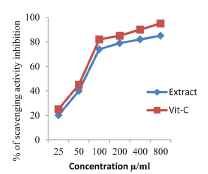
In H2O2 scavenging in vitro method, maximum free radical scavenging activity was shown at 800 μg/mL to a maximum of 86% and vitamin C at the same concentration showed 99% of inhibition [Table/Fig-3].
FRAP Assay
In FRAP assay here, extract shown inhibition 20% at 25 μ g/mL and a maximum 85% inhibition at 800 μg/mL and vitamin C at 800 μg/mL showed 95% inhibition [Table/Fig-4].
Nephroprotective Activity
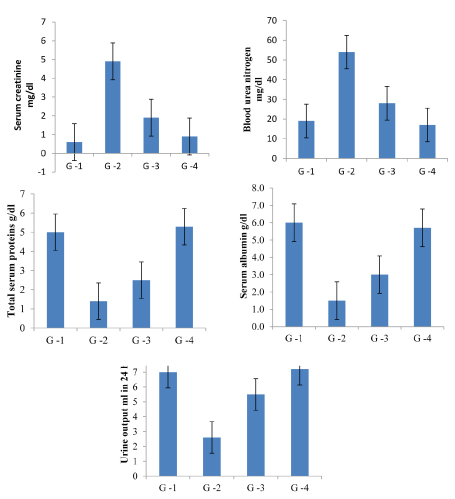
Results showed increased levels of serum creatinine, BUN and decreased total proteins, albumin and urine output in glycerol treated animals (group-2) compared with normal saline treated animals (group-1). Attenuation of serum creatinine, blood urea nitrogen and increased total proteins, albumin and urine output in glycerol + EEAHR 250 mg/kg treated animals (group-3) and glycerol + EEAHR 500 mg/kg treated animals (group 4). The changes were highly significant with group 4 than group 3. [Table/Fig-5] Serum creatinine, [Table/Fig-6] BUN, [Table/Fig-7] Total Proteins, [Table/Fig-8] Albumin and [Table/Fig-9] Urine output.
Showing the nephroprotective activity fo Aconitum heterophyllum root, among the four groups.
Histopathology
The horizontal section of rat kidney was showing normal glomeruli with an intact bowman’s capsule, proximal convoluted and distal convoluted tubules in the control group (group:-1). In glycerol (group:-2) treated rat was showing severe haemorrhagic and hyaline casts, capillary congestion, interstitial damage and apical blebbing. In animals treated with 250 mg/kg of EEAHR (group:-3) the kidney damage was found to be less than glycerol treated group. In animals treated with 500mg/kg of EEAHR (group:-4) the kidney damage was far less compared with 250 mg/kg of EEAHR [Table/Fig-10].
All figures are in 10x magnification. In group -1 the arrows have shown normal features and group-2 the arrows have shown abnormal features.
Group-1 (control):- Kidney was showing normal and well defined Glomeruli (G) with an Intact Bowman’s Capsule (IBC), Proximal and Distal convoluted tubules (PCT, DCT)) and Intistitium (I).
Group-2 (Glycerol):- It was showing severe hemorrhagic, hyaline casts, capillary congestion, interstitial damage, apical blebbing and damaged proximal and distal convoluted tubules
Group-3 (Glycerol + 250 mg/kg of extract):- The kidney damage was found to be less than the glycerol treated group
Group-4 (Glycerol + 500 mg/kg of extract):- The kidney damage was far less compared with 250 mg/kg of extract.

Discussion
AKI is becoming prevalent in developing and developed countries. Cerdá J stated that AKI takes place predominantly in urban intensive care units and is associated with multiorgan failure and sepsis, high mortality and occurrence in older populations [38]. The world-wide socioeconomic impact of AKI and it is due to the lack of specific treatments; necessitate major efforts to identify novel and effective therapeutic strategies. The mechanism of action of glycerol-induced ARF is characterized by myoglobinuria, tubular necrosis, cast formation, cytotoxicity and enhanced renal vasoconstriction caused by myoglobin, and cytokines released after rhabdomyolysis [39]. To study the acute effects this is one of the good model, as Glycerol induces changes within 24 h.
In this study, intramuscular injection of glycerol lead to markedly increased levels of serum creatinine and BUN and reduced the total proteins, albumins and urine output. The histological pattern of glycerol-treated rats showed characteristic haemorrhagic, hyaline cast deposits, tubular necrosis, capillary congestion and apical blebbing. The EEAHR dose of 250 mg/kg and 500 mg/kg of animals showed attenuation of all these changes. The changes were more significant with 500 mg/kg than 250 mg/kg. Treatment with EEAHR reduces the glycerol induced changes such as breakdown of muscle protein. This can be manifested by a decrease in serum creatinine, BUN and increase in total proteins and albumin. The urine output became normal in group 4 animals.
Histopathological changes of the kidney including marked capillary congestion, crust formation, tubular necrosis and inflammation and congestive changes are altered by the treatment. This indicates the possible protective effect of EEAHR against glycerol induced nephrotoxicity.
Glycerol induces damage by oxidative stress, apoptosis, and inflammation is key mediator of renal dysfunction [40–45]. The association between oxidative stress and nephrotoxicity has been well established in many experimental animal models. Nowadays, the research in the area of therapeutic potentials of medicinal plants as antioxidants in reducing tissue injuries caused by free radicals has been increasing.
In vitro antioxidant activity tested showed that it has good antioxidant and free radical scavenging actions. The EEAHR showed a protective effect probably due to its antioxidant activity. Ethanolic extract of A. heterophyllum contains alkaloids, steroids, saponins, glycoside and phenolic compounds [46]. The phytochemical constituents of root were reported that to be having stabilizing on the lysosomal membrane both in vitro and In vivo are flavonoids, tannins and saponins [47,48]. Freidelin (terpinoid) present in the plant leaves is found to have antioxidant, free radical scavenging and hepatoprotectiive [49]. The nephroprotective activity of the plant may be due to its antioxidant activity which is contributed by its phytochemical constituent stanina, phenolic compounds and saponins. Further research is needed to identify the exact bioactive compound responsible for this effect. In future taking these findings is necessary to develop new drugs in the treatment of renal diseases.
Conclusion
The results of these screening investigations have shown the EEAHR was found to have an in vitro antioxidant activity and protective effect in glycerol induced ARF in Wistar albino rats. The antioxidant activity and phytochemical constituents could have contributed to nephroprotective activity.