Tertiary Care Hospitals have become prime source and are conducive for the development and spread of antibiotic drug resistance [9,10]. Irrational antibiotic usage is a potential risk factor for increased worldwide emergence of acquired resistance by gram negative organisms [11–13]. Evolution of drug resistant mystery bugs had resulted in paramount cost burden [14], increased duration of stay, discontinuation of job, poor family economy, increase in morbidity and mortality by enlarge poses a huge menace to the community. Judicious and rational optimized use [15] of antibiotics by health care physicians is warranted to counter-turmoil this erratic situation. Only a few clinical audit reports were published in India and also in abroad targeting adherence to antibiotic policy for therapeutic management of drug resistant bacterial infections. So, a systematic and streamlined review [16] evaluation in the form of cross-sectional clinical audit to enhance the diagnostic and therapeutic facility for providing quality patient care was planned to combat the alarming multi drug resistant bugs. In-patients diagnosed with Urinary Escherichia coli ESBL as per medical case record were subjected to evaluate the compliance on appropriate antibiotic prescription and strict adherence to Hospital Antibiotic Policy for therapeutic management by the physician following the issue of Urine Culture and Antibiotic Susceptibility Test (AST) reports by counter-checking. In-patients of various departments like Medicine, Orthopedics and Surgery who were provisionally diagnosed to have UTIs were included in the study.
Materials and Methods
Patients
Between November 2014 and May 2015 (7 months), we conducted a prospective cross-sectional clinico-medical audit on adherence to treatment of Escherichia coli ESBL producers from in-patients diagnosed to have UTI.
Standards
Fluoroquinolones (FQ’s) will be the drug of choice for treating 100% of Escherichia coli Extended Spectrum b Lactamase (ESBL) producers in urine as per Hospital Antibiotic Policy (HAP) 2013-2014 of Karpagam Faculty of Medical Sciences & Research (KFMS&R). This policy was framed, based on Infectious Diseases Society of America (IDSA) practice guidelines which were formulated in collaboration with European Society of Clinical Microbiology & Infectious Diseases (ESCMID) [15]. Source of evidence: Hospital Antibiotic policy of KFMS&R: 2013-2014.
Study was conducted among the in-patients of Karpagam Medical College Hospital affiliated to Karpagam Faculty of Medical Sciences & Research (KFMS&R). A total of 164 medical case records of patients 18-50 years of age with urinary Escherichia coli ESBL infection were included in the study and the duration of study was seven months from November 2014 to May 2015. Urinary Escherichia coli ESBL producers were identified by standard tests (Culture on Blood Agar & Mac Conkey Agar Plate) and their Antibiotic Susceptibility Profiles (on Mueller Hinton Agar by Kirby – Bauer disk diffusion method) were also done and documented in Diagnostic Microbiology Laboratory. Screening test for ESBL was done based on Zone size between Ceftazidime (Ca) and Ceftazidime-clavulanate (CaC) where a difference of ≥ 5 mm is considered as suspected ESBL. The confirmative test for ESBL was done using Cefotaxime (Ce) and Cefotaxime-clavulanate (CeC) and a zone difference of ≥ 5 mm is used to diagnose ESBL producers. The patterns of antibiotic susceptibility were noted. Patient’s medical case records were looked for the correct antibiotic prescription or change in the prescription of the antibiotic after the issue of the culture report as per Hospital Antibiotic policy for treatment of urinary Escherichia coli ESBLs. Comparative evaluation of Microbiology laboratory report (Culture and Sensitivity) and patient treatment chart was assessed in the Medical case records of the patients maintained in the respective wards or in Medical records Department after the discharge of the patient. Compliance of appropriate antibiotic prescription and strict adherence to Hospital Antibiotic Policy was evaluated and results noted and the data was analysed.
Statistical Analysis
Data were analysed using IBM SPSS version 20 software.
Results
In the present study, 164 patients with urinary Escherichia coli ESBL were included. Male to female sex ratio was 0.86 with mild female pre-ponderance. The study population belonged to adult age group of 18 years to 50 years. The incidence of uncomplicated cystitis, pyelonephritis and complicated pyelonephritis cases were 65.24% (107 out of 164), 20.7% (34 out of 164) and 14.02% (23 out of 164) respectively. About 18.9% (31 out of 164 cases) were admitted in Intensive care unit (ICU) and remaining 81.1% (133 out of 164 patients) were admitted in Medicine, surgical and orthopedic wards. A total of 51.21% (84 out of 164) of the cases were empirically treated with Extended Spectrum Cephalosporins and Carbapenems due to associated co-morbid conditions like diabetes mellitus which may be due to antibiotic overuse of a particular class of drug. Incidence of Diabetes mellitus was found to be 16.46% (27 out of 164 patients). Among the isolates of urinary Escherichia coli ESBL producers, Nitrofurantoin was sensitive in 78.7% (129/164) of the cases.
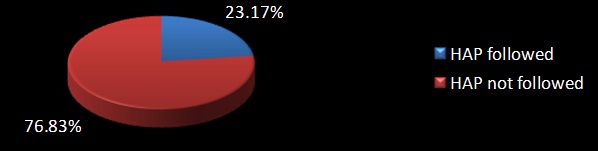
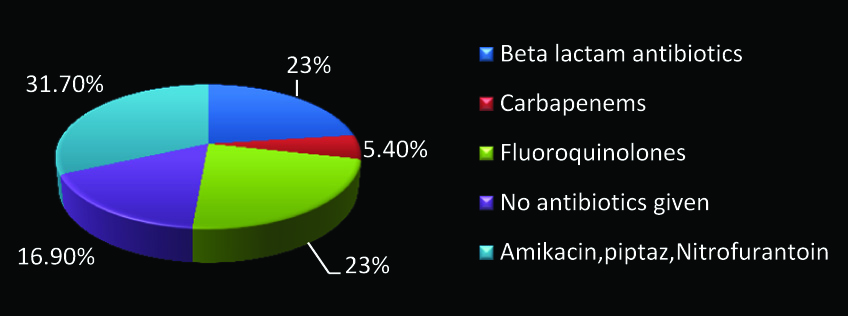
Antibiotics were changed by treating physician after issue of culture reports only in 43% (71 out of 164 patients). In that only 54.9% (39 out of 71) of the patients with Escherichia coli Extended β Lactamase Producers (ESBLs) in urine, had been given the right antibiotic in accordance with the antibiotic policy of our hospital. Around 14.63% (24 out of 164 cases) were given antibiotics before the issue of the Antibiotic Susceptibility Testing (AST) report and most of them were prophylactically given intravenous Ceftriaxone. Among them HAP was followed in 50% (12 out of 24 cases). Though 33.54% (55 out of 164 patients) were treated for urinary Escherichia coli ESBL with a sensitive drug following the issue of Antibiotic susceptibility testing report, only 23.17% (38 out of 164) of the patients were treated appropriately with fluoroquinolone as per Hospital antibiotic policy [Table/Fig-1]. Treatment with sensitive antibiotics included Tab. Nitrofurantoin which was used in 23.63% cases (13 out of 55), Inj. Levofloxacin in 16% (9 out of 55), Inj. Amikacin in 27.27% (15 out of 55), Piperacillin-Tazobactum in 25.45% (14 out of 55), Inj. Imipenem in 3.63% (2 out of 55) and Inj. Meropenem in 3.63% (2 out of 55) of the patients. Patient outcome at the time of discharge revealed the following treatment outcomes. Patient improved in 94.5% (155/164), Cured in about 0.6% (1/164) cases, no improvement in 1.2% (2/164), Outcome unknown in 2.4% (4/164) and patient got discharged Against Medical Advice (AMA) in 1.8% (3/164). Surprisingly, no mortality was observed among 164 patients in this study, the exact reasons were unknown. Even though resistance to Amoxicillin, Amoxicillin-clavulanate and Ceftriaxone was 100% by AST as depicted in [Table/Fig-2], 23% (38 out of 164) of the patients were treated with Ceftriaxone. One major concern is that the use of Ceftriaxone in ESBL patients can accelerate the development and spread of resistance. Amikacin was found to be sensitive in almost all the patients. Distribution of treatment strategies for treating urinary Escherichia coli ESBLs by physicians is depicted in [Table/Fig-3].
HAP followed in treatment of urinary Escherichia coli ESBLs. Urinary Escherichia coli ESBLs treated with Fluoroquinolones given as per HAP (N=164).
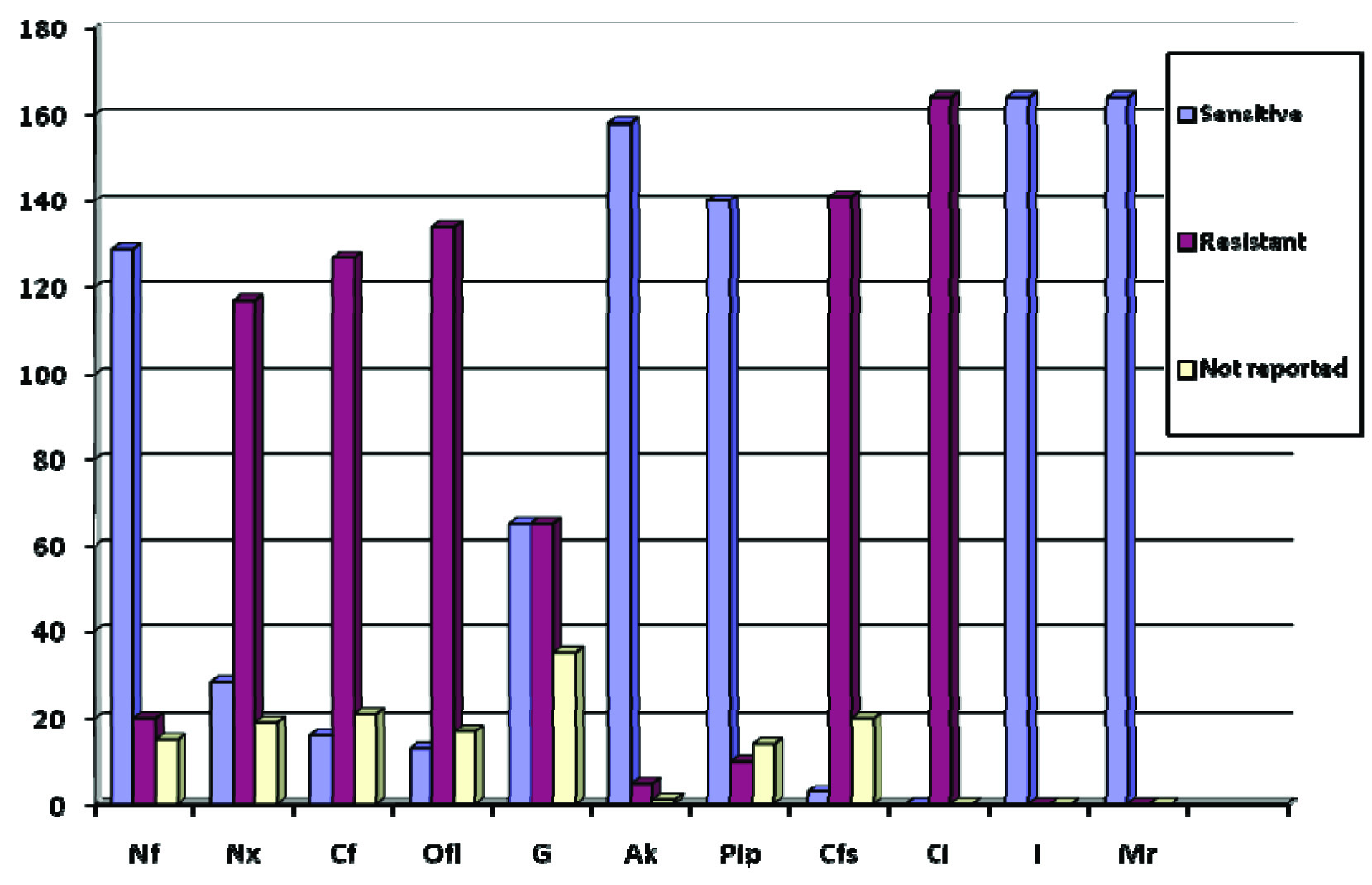
AST pattern of 164 clinical isolates of Urinary Escherichia coli ESBL.
Nf- Nitrofurantoin, Nx-Norfloxacin, Cf-Ciprofloxacin, Ofl-Ofloxacin, G-Gentamicin, Ak-Amikacin, Pip-Piperacillin-Tazobactum, Cfs-Cefoperazone-Sulbactum, Ci-Ceftriaxone, I-Imipenem, Mr-Meropenem.
Distribution of treatment strategies for treating urinary Escherichia coli ESBLs by physicians (N=164).
Discussion
Most common cause of UTI is Escherichia coli followed by Klebsiella pneumoniae. Generally community acquired UTI are treated with Cotrimoxazole (Trimethoprim-Sulfamethoxazole). Hospital acquired UTI are treated with first or second generation cephalosporins but in the recent times most of these bacteria had developed resistance to a broad spectrum β lactam drugs by producing Extended Spectrum β Lactamase (ESBL) enzymes. Escherichia coli ESBL producers had emerged as a dangerous nosocomial pathogen causing significant morbidity and mortality among patients. Culture-based methods still remain as the cornerstone for diagnosis and detection of antibiotic resistance. Early detection followed appropriate medical therapy may be helpful in the management of ESBL producing Escherichia coli in UTI. Therapeutic options for ESBL producing Escherichia coli in urine is to treat the patients with Nitrofurantoin, Fluoroquinolones (Norfloxacin, Ciprofloxacin, Ofloxacin, etc.), Carbapenems (Imipenem, Meropenem or Ertapenem), Fosfomycin, Mecillinam or Tigecycline. ESBL production in Escherichia coli could be a risk factor for development of Carbapenemase resistance in the near future. A detailed review and guideline evaluation was a necessity as it was never conducted despite overgrowing antimicrobial resistance [16]. Prompt measures to prevent the spread of such drug resistant bugs by proper sterilization, disinfection and hand washing practices and also by adherence to hospital antibiotic policy would prophylactically prevent the incidence of iatrogenic nosocomial infections which is common.
In this study, antibiotics were changed in 43% (71 out of 164 cases). In that only 54.9% (39 out of 71) of the patients with Escherichia coli Extended B Lactamase Producers (ESBLs) in urine, had been given the right antibiotic in accordance with the antibiotic policy of our hospital. Around 15.75% (24 out of 164 cases) were empirically given antibiotics before the issue of the Antibiotic Susceptibility Testing (AST) report and most of them were prophylactically given intravenous Ceftriaxone. This was far better compared to a study conducted in Aberdeen which reported significant empirical overuse [17] leading to high mortality. Similar reports were seen in studies conducted in China [18], New York [19], Malaysia [20], Thailand [21] and South Africa [22]. Hospital antibiotic policy was followed in only 50% (12 out of 24) of the cases. Nearly 94% (155 out of 164) improved with or without treatment, but cure rate was just 0.6%. Approximately 17% (28 out of 164) of patients did not receive any antibiotic during their stay in the hospital due to various reasons. About 1.8% of cases were discharged against medical advice (3 out of 164) without completing specific medical therapy. Antibiotics were not changed after AST report and antibiotic policy was not followed in about 32.72% (54 out of 164) of the cases, it could be due to lack of knowledge of physician about management of ESBLs.
Appropriate prescription as per hospital antibiotic policy was followed only in 23% cases (39 out of 164) of the patients. Rational non-adherence to antibiotic policy in treating ESBL producing Escherichia coli can occur due to various confounding factors like fear of drug toxicity and or drug allergy in the patients, in-vitro resistance of specific antibiotics, affordability to buy antibiotics, non-availability of specific broad or extended spectrum antibiotics in the market. About 25% (41 out of 164) patients were treated with more than one antibiotic for the associated conditions. Duration of therapy with intravenous fluoroquinolone antibiotics varied from one day to maximum of 8 days which were then switched to oral therapy at the time of discharge. Resistance to individual fluoroquinolones like Norfloxacin, Ciprofloxacin and Ofloxacin were found to be 60%, 59% and 47.5% respectively which was twice the resistance rate reported in another study conducted abroad [23]. Aminoglycosides like Amikacin and Gentamicin showed sensitivity of 96% and 39% respectively where the resistant rates to aminoglycosides were less compared to another study conducted elsewhere. Amoxicillin, amoxicillin-clavulanate and ceftriaxone showed 100% resistance in the subjects. Simultaneously sensitivity of carbapenems like Imipenem and Meropenem were 100% which were similar to a study conducted in Phillipines [23]. Piperacillin-tazobactum was sensitive around 85%. Increasing fluoroquinolone resistance may be a reason for not being the only antibiotic of choice for Escherichia coli ESBLs. Antibiotics like Fosfomycin, Nitrofurantoin, Piperacillin-tazobactum, piv-mecillinam, Carbapenems (Imipenem, Meropenem, Ertapenem, Doripenem), Temocillin [24,25], Colistin and Tigecycline [26] can be appropriately used as alternative drug of choice for treatment of Fluoroquinolone resistant ESBL producing Escherichia coli. Nitrofurantoin were sensitive in 59% as per AST but only 13 out of 55 patients (23%) had been given Nitrofurantoin and the reasons were unknown.
Limitations
This study includes the following limitations: Unavailability of permanent surveillance system to monitor bacterial resistance to antibiotics; Tracking up of patients diagnosed with Escherichia coli ESBL from Microbiology lab to the ward; Lack of integrated and uninterrupted co-operation from the physicians, nurses and Medical Records Department staffs; Round the clock evaluation of patient treatment chart; Improper as well as incomplete culture and sensitivity reports; Poorly filled patient medical case records; Delay in reports followed by delay in treatment; Losing track of patients resulting due to unexpected discharge from the hospital; Inappropriate adherence to Hospital Antibiotic Policy due to insufficient knowledge and awareness of physician in early detection and initiation of right medical therapy; Lack of Microbiology facility; Patient’s expectation to receive antibiotic irrespective of the necessity; Physician’s practice to prescribe unnecessary antibiotics to any patient; Material gain for pharmacist from selling the antibacterial antibiotics; Patient’s unawareness about right use of antibiotics.
Improper sterilization and disinfection practices and hand washing techniques, irrational antibiotic usage, non-adherence to hospital antibiotic policyand etc., could have had possibly accounted for increased antibiotic resistance as a result of ‘Emerging Drug resistant bio-burden’ in this study. Stringent therapeutic guidelines, Protocols for rational therapeutics and hospital or institutional antibiotic policy must be available and updated periodically so as to provide a right platform for treating physicians. Conduction of regular clinical audits on adherence to antibiotic policy, practice of universal safety precautions, rational use of antibiotic drugs, appropriate antibiotic selection during treatment of Escherichia coli ESBL producers are mandatory to maintain uniformity and establish clinical standard in giving Quality patient care. Treating physicians, microbiologists, nurses and pharmacists should be educated about ESBL producing bacteria, their diagnosis and therapeutic management like Antibiotic of choice, dosage, Schedule, contra-indications to any drug for treating Urinary Escherichia coli ESBLs as per Hospital Antibiotic Policy of the hospital. Implementing the change of antibiotic per hospital antibiotic policy especially when treated with β lactam antibiotics following report of ESBL Escherichia coli is warranted. Periodic auditing regarding sustenance of the Antibiotic policy norms has to be conducted once in every 3 months. Stringent sterilization and disinfection practices, meticulous hand washing to be strictly followed by health care personnel to prevent contact transmission. Regular and periodic update of Hospital Antibiotic Policy as per revised IDSA guidelines should be implemented. Periodic surveillance on screening by culture isolation and antibiogram of microbial pathogens prevailing in the hospital environment should be undertaken and the results discussed among clinician. Hospital Infection Control Committee (HICC) meeting must be held once in every 3 months period to sustain and or enhance the effectiveness of Hospital Infection Control.
Conclusion
First clinical audit from Southern India reflects the need for strict adherence to hospital antibiotic policy guidelines in case of antibiotic selection and treatment of Urinary Escherichia coli Extended Spectrum β Lactamases by the physicians which is the key to control the disease spread and cost burden to patients worldwide. Irrational utilization of antibiotics and non-adherence to antibiotic policy are the significant risk factors for drug resistance. Optimized antibiotic use assisted by effective infection control team and Microbiology laboratory support would regulate rational drug use, cut cost and will slow down the emergence of antibiotic resistance. Need for constituting an antibiotic monitoring team consisting of Medical Microbiologist, Physician, Infection control staff, Pharmacist and MRD in-charge for periodic surveillance on early diagnosis, susceptibility pattern, treatment, prevention and adherence to antibiotic policy for Escherichia coli ESBL infections is mandatory so as to provide a quality patient care.