Spinal anaesthesia is the gold standard for lower abdominal surgeries. It has got the advantage of being, cost-effective, easy administration technique, rapid onset of action, with relatively less adverse effects and most importantly patient remaining aroused throughout the procedure [1]. But at times short duration and uncomfortable postoperative period offset the above advantages. Therefore, in order to extend the intraoperative analgesia into postoperative period, following spinal anaesthesia, various spinal adjuvants like morphine, buprenorphine and fentanyl, clonidine, ketamine are being used in anaesthetic practice. Such adjuvants have been helpful in induction of early ambulation along with prolongation of analgesia but at the cost of their associated adverse effects. Therefore search for an effective adjuvant is still going on.
In this context, the present study was designed to evaluate the efficacy of intrathecal Dexmedetomidine as an adjuvant to Bupivacaine in spinal anaesthesia in patients undergoing infra-umbilical surgeries.
Materials and Methods
It was a prospective, double blind study among 60 adult patients of either sex, in the age group of 18-45 years. Prior approval from the Institutional Ethics Committee and written informed consent from the enrolled patients was obtained.
Inclusion criteria
Patients selected for elective infra-umbilical surgeries of approximately one and half hour (90minutes) duration under spinal anaesthesia.
Patients who belong to American Society of Anaesthesiologists (ASA) physical status grade I and II.
Exclusion criteria
Patients with coagulation disorders, neurological disorders.
Patients allergic to study medication.
Patients showing unwillingness for spinal anaesthesia.
Settings and Design: The patients were randomly allocated to 2 groups (Group I and Group II) of 30 each. Group I received 3 ml of 0.5% Hyperbaric Bupivacaine (15 mg) and 0.5 ml normal saline (total 3.5ml). Group II received 3 ml of 0.5% Hyperbaric Bupivacaine (15 mg) with 5 μg Dexemedetomidine made up to 0.5 ml with normal saline (total 3.5 ml).
Visual Analogue Scale (VAS) was used as the study instrument to determine the level of analgesia postoperatively [4]. During the pre-anaesthetic checkup patients were explained about VAS and the level of analgesia in the postoperative period was recorded with score 0 indicating no pain and 10 indicating severe pain. They were advised fasting for 6 hours prior to the surgery and prescribed tablet Alprazolam 0.5 mg as premedication.
Baseline parameters like pulse, blood pressure; ECG was measured before the surgical procedure. Intravenous line was secured and patients were preloaded with Ringer’s lactate 10 ml/kg body weight over 20 to 30 minutes. They were randomly allocated into the two groups using sealed envelope technique by a person blinded to the procedure. The study medication was prepared by an anaesthesiologist not involved in the study and another anaesthesiologist performing the spinal block recorded the intraoperative and postoperative data.
Lumbar puncture was performed under aseptic conditions, in left lateral position by midline approach by using Quincke spinal needle (25G) at L3-L4 intervertebral space. Total volume of 3.5 ml of drug was deposited in subarachnoid space after assuring the free flow of clear Cerebrospinal Fluid. Immediately following the injection, patients were made to lie in the supine position. Continuous monitoring of haemodynamic parameters was done and readings were recorded every 5 minutes for first 30 minutes, thereafter every 10 minutes till the end of surgery.
The onset of sensory block was tested by ‘pin-prick method’ using a hypodermic needle at 2 minutes interval. The time of onset was taken from the time of injection of drug into subarachnoid space to loss of pinprick sensation. The highest level of sensory block, time for two dermatomal segments regression of sensory level, duration of sensory blockade taken as time from onset to time of return of pinprick sensation to S1(heel) dermatomal area were noted. VAS was recorded at an interval of 3, 6, 12 hours postoperatively. Duration of complete analgesia was noted and rescue analgesics were given to the patients when VAS >3. The cutoff point of our study was the time when the patient demanded the first dose of rescue analgesia.
The onset of motor block was assessed with ‘Modified Bromage Score’. The time interval between injections of drug into subarachnoid space, to the patient’s inability to lift the straight extended leg was taken as onset time (Bromage 3). The duration of motor block was taken from time of injection to complete regression of motor block (ability to lift the extended leg) (Bromage 0).
Modified Bromage Scale [5]:
Grade0- Full flexion of knees and feet.
Grade1- Just able to flex knees, full flexion of feet.
Grade2- Unable to flex knees, but some flexion of feet possible.
Grade3- Unable to move legs or feet.
Sedation scores were assessed every 15 minutes intraoperatively using a ‘four point score’ [6]: 0 – No sedation, 1 – mild sedation, 2 – moderate sedation, 3 – deep sedation.
Side effects like sedation, nausea, vomiting, shivering were monitored in the recovery room and then patient shifted to the ward.
Statistical Analysis
Data obtained were tabulated and analysed using Graph Pad prism 6.0 software. Continuous data were analysed using the Student’s t-test and categorical variables by two-tailed Fisher-exact test or Chi-square test. All values were expressed as percentage or Mean±Standard deviation. The p< 0.05 was considered statistically significant.
Results
A total of 60 patients were enrolled in the study with 30 in each group. Both the groups were comparable with respect to the demographic parameters like age, sex, weight, height, duration and type of surgery [Table/Fig-1]. The infra abdominal surgeries which the patients had under gone were hysterectomy, hernia repairs, appendicectomy and open urosurgical procedures.
| Parameter | Group II | Group I |
|---|
| Age (Y) | 35.3±7.07 | 31.7±8.46 |
| Male: Female | 18:12 | 18:12 |
| Height (Ft) | 5.49±0.28 | 5.52±0.31 |
| Weight (Kg) | 54.10±7.33 | 56.93±8.48 |
Data represented as Mean±SD for various parameters.
The mean time of onset of sensory block in Group II was 129.33±14.84 seconds and in Group I it was 208.33±19.18 seconds, which was significantly (p<0.001) faster in Group II as compared to Group I. The mean time for onset of motor block was also statistically significant in Group I (320.33±29.81 seconds) as compared to Group II (226.33±31.86 seconds) [Table/Fig-2].
Onset of sensory and motor block (in seconds).
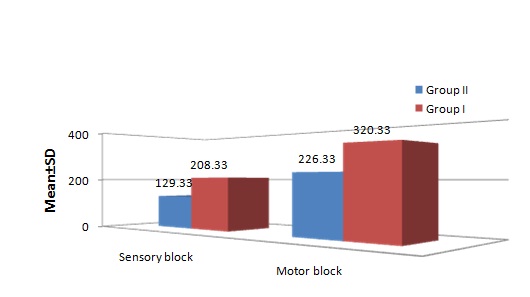
The time of two segment regression was considerably slower in Group II with 126.8±5.6 minutes compared to Group I which was 88.97±8.57 minutes. The difference was statistically significant (p<0.001). The mean duration of sensory block in Group II was 317.7±16.16 minutes and in Group I was 188±11.86 minutes. Mean duration of motor recovery in Group II was 286.33±15.15 minutes and Group I was 166.5±12.11 minutes which was also statistically significant (p<0.001) [Table/Fig-3].
| Recovery parameters (min) | Group II | Group I |
|---|
| Time to 2 segment regression | 126.80±5.60* | 88.97±8.57 |
| Time to complete sensory recovery | 317.70±16.16* | 188±11.86 |
| Time to complete motor recovery | 286.33±15.15* | 166.5±12.11 |
Data represented as Mean±SD. * ⇒ p< 0.001
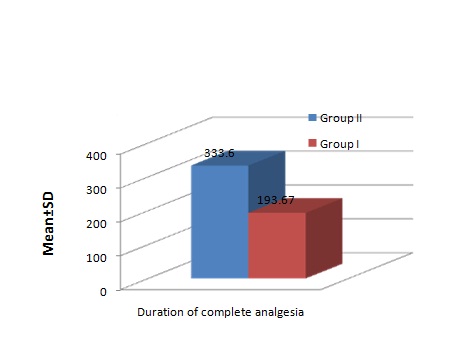
A statistically significant difference in duration of complete analgesia was observed between the two groups (Group II 333.6±20.6 minutes and Group I 193.67±7.06 minutes [Table/Fig-4].
Duration of Analgesia (min).
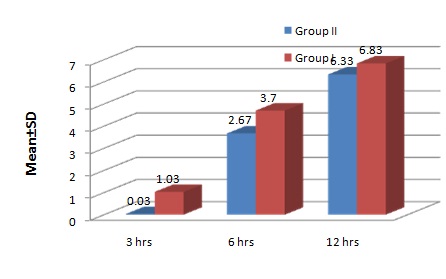
Intraoperative Visual Analogue Score was <3 in both the groups. At the end of 3 hours postoperatively, it was 0.03 and 1.03 in Group II and Group I respectively. But at the end of 6 hours VAS was 2.67 and 3.7 in Group II and Group I respectively where rescue medication was started for Group I. Twelve hours postoperatively the scores were 6.3 and 6.8 in Group II and Group I respectively. VAS values were significantly lower up to 3 and 6 hours postoperatively in Group II implying patients had better pain relief in the postoperative period than in Group I [Table/Fig-5,6].
Visual Analogue Scale (VAS) Scores Postoperatively.
| TIME | GROUP–II | GROUP–I |
|---|
| 3hours | 0.03±0.18 | 1.03±0.99 |
| 6hours | 2.67±0.88* | 3.70±1.15 |
| 12hours | 6.33±0.92 | 6.83±0.91 |
Data represented as Mean±SD. * ⇒ p< 0.001
Visual Analogue Scale (VAS) score postoperatively.
The two groups differ significantly with respect to the haemodynamic parameters. Heart rate was measured at interval of 0, 5, 10, 15, 20, 30 minutes. Group II patients had mean heart rate 66.7, 66.63, 69.7 at 15, 20, 30 minutes and Group I patients had mean heart rate 72.67, 74.47, 76.47 at 15, 20, 30 minutes respectively which was statistically significant (p<0.05) [Table/Fig-7].
| Time Interva(Minutes) | Heart Rate | SBP (mm of Hg) | DBP (mm of Hg) |
|---|
| Group II | Group I | Group II | Group I | Group II | Group I |
|---|
| 0 | 77.13±6.5 | 80.63±8.08 | 127.27±11.5 | 126.20±9.8 | 79.33±6.9 | 77.26±6.4 |
| 5 | 74.23±7.0 | 78.73±10.79 | 119.43±11.49 | 116.30±9.9 | 74.53±8.7 | 72.83±7.7 |
| 10 | 72.83±8.5 | 75.70±7.31 | 107.13±9.17 | 107.53±8.9 | 65.73±7.4 | 66.07±6.6 |
| 15 | 66.70±7.9 | 72.67±7.4 | 101.67±7.26 | 106.10±10.4 | 64.67±6.5 | 67.37±7.1 |
| 20 | 66.63±7.5 | 74.47±8.5 | 105.13±5.9 | 108±9.1 | 62.53±5.7 | 68.07±6.8 |
| 30 | 69.70±5.8 | 76.47±7.15 | 108.5±8.2 | 113.73±7.4 | 68.63±5.5 | 70.87±5.5 |
| 120 | 75.33±5.1 | 77.86±5.8 | 118.47±7.9 | 120.9±7.3 | 74.67±4.8 | 74.27±6.4 |
Data represented as Mean±SD.
The mean systolic blood pressure (SBP) in Group II decreased from baseline 127.27 mmHg to 119.43 mmHg at 5 minutes,107.13 mmHg at 10 minutes,101.67 mmHg at 15 minutes,105.13 mmHg at 20 minutes,108.5 mmHg at 30 minutes and gradually increased to 118.47 mmHg at the end of 2 hours. The mean systolic blood pressure (SBP) in Group I also decreased from baseline 126.20 mmHg to 116.3 mmHg at 5 minutes,107.53 mmHg at 10 minutes,106.10 mmHg at 15 minutes,108 mmHg at 20 minutes,113.73 mmHg at 30 minutes and gradually increased to 120.9 mmHg at the end of 2 hours. Hence the changes in SBP at any interval were not significant [Table/Fig-7].
The mean diastolic blood pressure (DBP) in Group II decreased from baseline 79.33 mmHg to 74.53 mmHg at 5 minutes,65.73 mmHg at 10 minutes, 64.67 mmHg at 15 minutes,62.53 mmHg at 20 minutes, 68.63 mmHg at 30 minutes and gradually increased to 74.67 mmHg at the end of 2 hours. The mean diastolic blood pressure (DBP) in Group I also decreased from baseline 77.26 mmHg to 72.83 mmHg at 5 minutes, 66.07 mmHg at 10 minutes, 67.37 mmHg at 15 minutes, 68.07 mmHg at 20 minutes, 70.87 mmHg at 30 minutes and gradually increased to 74.27 mmHg at the end of 2 hours. Hence the changes in DBP at any interval were also not significant [Table/Fig-7].
In Group II, 9.99% patient experienced hypotension, 9.99% had bradycardia, 3.33% had nausea/vomiting and 3.33% shivering when compared to Group I in which 9.99% had hypotension, 6.66% had bradycardia, 6.66% had nausea/ vomiting and 6.66% had shivering [Table/Fig-8]. There was no respiratory depression and sedation in both the groups.
| Adverse effects | Group II n (%) | Group I n (%) |
|---|
| Nausea/vomiting | 1 (3.33) | 2 (6.66) |
| Bradycardia | 3 (9.99) | 2 (6.66) |
| Sedation | 0 | 0 |
| Hypotension | 3 (9.99) | 3 (9.99) |
| Respiratory depression | 0 | 0 |
| Shivering | 1(3.33) | 2(6.66) |
Discussion
α-2-adrenoceptor agonists are being currently explored in anaesthetic field for their sedative, analgesic, sympatholytic, anaesthetic-sparing and favorable haemodynamic properties. Dexmedetomidine, is one such agonist having a relatively high α2/α1-activity ratio (1620:1) as compared to clonidine (220:1). It’s also unique that it lacks respiratory depressant action, and conscious sedation making it therapeutically a useful and safe adjunct [2,7].
In the spinal cord, activation of both α2 C and α2-a adrenoceptors, located in superficial dorsal horn neurons especially the lamina II, directly reduces pain transmission by reducing the release of pro-nociceptive transmitters, substance and glutamate from primary afferent terminals and by hyperpolarizing spinal interneurons via G-protein-mediated activation of potassium channels. Postsynaptic activation of central α2 adrenoceptors, results in a fall in blood pressure and heart rate, which has been clinically exploited to attenuate surgical stress [8].
In our study, both groups were comparable with respect to demographic profile, duration and type of surgery. The primary outcome of present study was early onset and increased duration of both sensory and motor block along with prolonged postoperative analgesia following addition of 5 mcg Dexmedetomidine to 15 mg of 0.5% hyperbaric Bupivacaine in spinal anaesthesia.
In the present study, there was statistically significant difference with regard to onset of sensory and motor block between both the groups with faster onset in Dexmedetomidine. Our results correlate with studies done by, Al-Mustafa et al., Sheriff A Abdelhamid et al., and Deepika Shukla et al., [9–11]. The time for 2 segment regression was considerably prolonged in Group II with 126.8±56 minutes and in Group I it was 88.9±8.5 minutes which is similar with the findings of Sheriff A Abdelhamid et al., Hala E A et al., GE Kanazi et al., Rajni Gupta et al., [9,12–14]. Similarly the duration of both sensory and motor blockade was significantly prolonged in Group II which is in agreement with studies done by the above authors.
Duration of analgesia was significantly prolonged in Group II (333.6min) as compared with Group I (193.6min) and also addition of Dexmedetomidine to intrathecal bupivacaine significantly decreased the requirement of rescue analgesia in the postoperative period [10,14].
In the present study, there was significant reduction in the VAS scores of the patients receiving Dexmedetomidine as compared with higher VAS scores in patients receiving bupivacaine alone in the six hours postoperatively. This implies better quality of analgesia postoperatively and reduced need of analgesics with the use of intrathecal Dexmedetomidine.
Cardiovascular profile in our patients was found to be remarkably stable throughout the intraoperative and postoperative period in both the groups so also was the adverse effect profile, which is in accordance with previous studies [10,13,15].
Dexmedetomidine is evolving as an efficacious analgesic by different routes and is varying doses in a variety of clinical situations. Findings of the present study add to its role as an intrathecal adjunct to bupivacaine in spinal anaesthesia.
Limitation
The present study was conducted on a small sample of population who were to undergo infra-umbilical surgeries within a two month study period. Further research for a longer duration and adequate sample size may substantiate the above findings.
Conclusion
Addition of 5mcg of Dexmedetomidine to 0.5% hyperbaric Bupivacaine 15mg (3.5mL) in spinal anaesthesia significantly decreases the onset time, prolongs the duration of both sensory and motor blockade, improves the quality of postoperative analgesia with better haemodynamic stability as compared to bupivacaine alone. Thus, the present comparative study concluded that ‘Addition of dexmedetomidine potentiates bupivacaine spinal anaesthesia’.
Data represented as Mean±SD for various parameters.
Data represented as Mean±SD. * ⇒ p< 0.001
Data represented as Mean±SD. * ⇒ p< 0.001
Data represented as Mean±SD.