The treatment of depression relies on various groups of antidepressants. Most significant of these are the Selective Serotonin Re-uptake Inhibitors (SSRIs). Fluoxetine is prototype of the SSRIs. It is longest acting drug, with plasma half life of 2 days. Fluoxetine is widely used for different psychiatric conditions in different doses and duration. SSRIs selectively inhibits membrane associated Serotonin Transporter (SERT) thus inhibits the 5–hydroxytryptamine (5-HT) (Serotonin) re-uptake. Increased synaptic availability of serotonin stimulates large number of postsynaptic (5-HT) receptor subtypes which lead to complex secondary responses. SSRIs are relatively safe and have better acceptability. These qualities of SSRIs have made them first line of drugs in depression [1–4]. It was observed that Growth (body weight gain, femur length) was decreased in animals which received high dose of SSRIs [5,6]. Psychiatry patients usually developed complaint of weight change during their illness and also during their recovery from illness. Clinical reports stated that fluoxetine causes weight gain. If a particular drug causes weight gain, patient may switch to other drug [7,8]. Some reports stated that fluoxetine causes weight loss; Even now-a-days fluoxetine is used and tested as a drug for weight loss [9,10]. According to Landry et al., 5-HT (2A) serotonin receptors produce some neuroendocrine responses which were responsible for weight loss [11]. Animal studies showed intrauterine growth retardation and premature birth after the administration of fluoxetine to female rats during gestation period [12,13].
The aim of this study was to find out whether fluoxetine causes weight gain or weight loss, and to deduce the comparative weight change after intraperitoneal injection of Fluoxetine for different duration and doses.
Materials and Methods
The duration of this observational study was one year from May 2009 to April 2010. The study was carried out in the Department of Anatomy, Himalayan Institute of Medical Sciences, Swami Ram Nagar, Dehradun. Total of 72 male and female albino rats of Rattus norwegicus strain weighing approximately 120-160 g were used in the present study. These were obtained from the Central Animal House of Himalayan Institute of Medical Sciences, Dehradun (Uttarakhand). The approval of IAEC (Institutional Animal Ethical Committee) was obtained before starting the study. All the albino rats were nutritionally healthy and free from any disease or disability. They were allowed access to food and water ad libitum throughout the experiment with standard balanced diet. The experimental animals were housed in cages with a 12 hour: 12 hour light – dark cycle. Present experimental animal study was carried out by using the drug fluoxetine hydrochloride (Cap. Flunil - 20mg manufactured by INTAS Pharmaceuticals). The drug was provided by the medical representative of manufacturing company.
The 20 mg drug – Fluoxetine hydrochloride was dissolved in 2 milliliters (ml) of normal saline so that each ml of normal saline had a drug concentration of 10 mg. The drug was injected intraperitoneally (I/P), according to weight of the rat, once in a day. Three phases of this study was of 2 weeks, 4 weeks and 12 weeks duration. Animals in each phase consisted of 24 male and female. These animals were further subdivided into 4 Groups of 6 albino rats each (3 males & 3 females) randomly. Six rats were of Group 1(Control) received normal saline (vehicle). Eighteen rats were experimental rats, of Group 2, Group 3 and Group 4 (6 rats each) which received 10mg/kg, 20 mg/kg and 40mg/kg of intraperitoneal injection of fluoxetine respectively. All the rats were weighed on each day in order to facilitate the dose calculation and growth monitoring. Changes in the body weights of the albino rats were recorded. Data was subjected to statistical analysis (Mean, standard deviation and Student’s t-Test).
Results
The initial mean body weight of Group 1 (control) rats for 2 weeks, 4 weeks and 12 weeks was 131 g, 126.7 and 140 g respectively. Their mean weight reached to 131.33 g, 145.67 g and 164.5 g at the end of 2 weeks, 4 weeks and 12 weeks respectively [Table/Fig-1,2].
Changes in the mean body weight of control and experimental albino rats.
| Groups | 2 weeks(Mean ± SD) | 4 weeks(Mean ± SD) | 12 weeks(Mean ± SD) |
|---|
| Group 1 (Control) | 1.74 ± 13.27 | 15.54 ± 8.77 | 17.80 ± 6.49 |
| Group 2 (10mg/kg/day) | -0.15 ± 9.86p=0.067 | -9.81 ± 0.22p=0.02 | -13.10 ± 7.21p=0.03 |
| Group 3 (20mg/kg/day) | -10.70 ± 4.77p=0.00152 | 7.83 ± 13.47p=0.13 | - 21.11 ± 10.05p=0.04 |
| Group 4 (40mg/kg/day) | -15.83 ± 9.57p=0.02 | - | - |
(p < 0.01 - Highly significant, p < 0.05 – Significant, p > 0.05 – Non significant)
Change in mean body weights in Controls and Experimental Groups of albino rats after administration of intraperitoneal injection of Fluoxetine
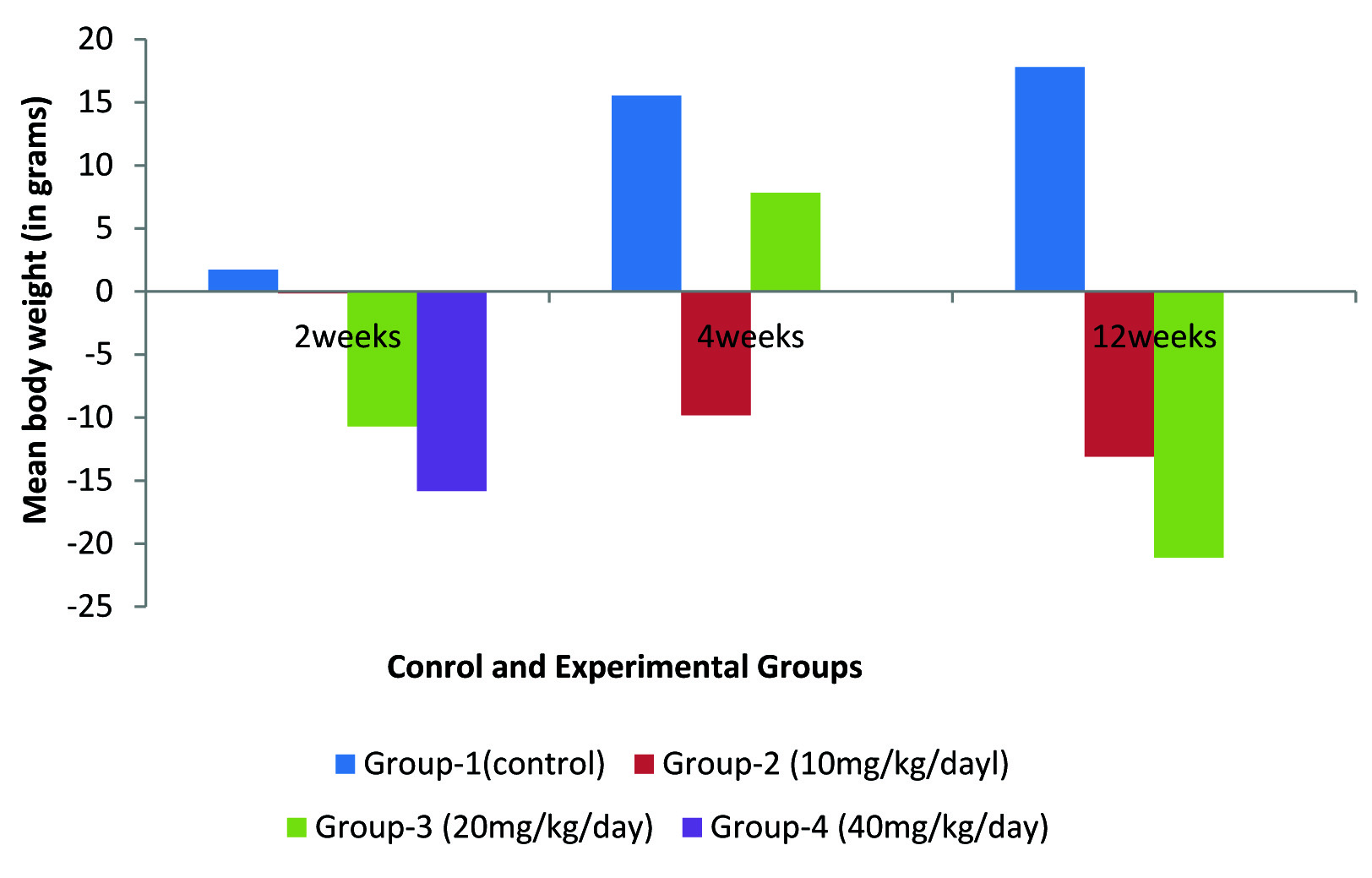
The initial mean body weight of Group 2 (10 mg/kg/day) experimental rats for 2 weeks, 4 weeks and 12 weeks was 150 g, 143.71 and 149 g respectively. Their mean weight reached to 147.33 g, 129.14 g and 129 g at the end of 2 weeks, 4 weeks and 12 weeks respectively [Table/Fig-1,2].
The initial mean body weight of Group 3 (20 mg/kg/day) experimental rats of 2 weeks, 4 weeks and 12 weeks was 127.2 g, 109.2 g and 113.33 g respectively. Their mean weight reached to 114 g, 117.6 g and 89.33 g at the end of 2 weeks, 4 weeks and 12 weeks respectively [Table/Fig-1,2].
The initial mean body weight of Group 4 experimental rats was 110 g. Group 4 (40 mg/kg/day) rats survived only up to 2 weeks. The rats of this group could not tolerate this dose for long period and gradually died. Those animals which survived up to 2 weeks showed a drastic decrease in the mean body weight by 15.83 g [Table/Fig-1,2].
The mean body weight of group 1 (control) albino rats increased by 1.74 ± 13.27 g after 2 weeks, 15.54 ± 8.77 g after 4 weeks and 17.80 ± 6.49 g after 12 weeks [Table/Fig-1,2].
The group 2 (10 mg/kg/day) experimental albino rats showed a slight decrease of weight by 0.15 ± 9.86 g. On continuous injection of fluoxetine for 4 weeks the mean body weight decreased by 9.81 ± 10.22 g. On further administration of the drug up to 12 weeks, the body weight showed an enormous decrease by 13.10 ± 7.21 g. The results at 4 and 12 weeks were significant (p value < 0.05) [Table/Fig-1,2].
Group 3 (20 mg/kg/day) experimental rats showed an initial decrease of body weight by 10.70 ± 4.77 g. At the end of 4 weeks the body weight increased by 7.83 ± 13.47 g which could probably be due to gradual adaptation of the rats to the drug. Finally, at the end of 12 weeks there was a huge decrease in the body weight of rats by 21.11 ± 10.05 g. All results were highly significant (p < 0.01) [Table/Fig-1,2].
Group 4 (40 mg/kg/day) experimental rats survived only upto 2 weeks but gradually the rats of this Group died featured with muscle twitching, sluggish body movements and loose stools. Those animals which survived up to 2 weeks showed a drastic decrease in the mean body weight by 15.83 ± 9.57 g (p < 0.05) [Table/Fig-1,2].
Discussion
The cause of depression can be lack of chemical serotonin. SSRIs maintain levels of serotonin in brain to correct depression. SSRIs have common side effects, including fatigue, weight loss, apathy, insomnia, dry mouth, nausea, headache, diarrhea, weight gain [14].
Norman sussman reported weight gain occur with use of SSRIs for more than 8 to 12 weeks. A review published in 2003 in the Cleveland Clinic Journal of Medicine stated that weight gain is a possible side effect with SSRIs; if used for more than six months or more. The SSRIs encourage carbohydrate craving that is hard to control. A group of Italian researchers observed that recovery from depression may play a role in weight gain [7,8].
Afkhami-Ardekani M and Sedghi H reported that weight loss was one of the frequently reported side effects of fluoxetine. But weight gain was also found in 1 or < 1 out of 1000 people. The studies on young rodents on growth showed an impairment of bone development/growth on exposure to fluoxetine. The impact of toxicity was mostly in juvenile rodents. A 19-week clinical trial showed 1.1cm decreased height (p=0.008) and weight loss in children and adolescents treated with fluoxetine. Weight loss was proportional to baseline body weight. Weight loss could be due to undesirable effects on digestive system such as diarrhea, nausea, vomiting, dyspepsia, dysphasia, taste perversion and dry mouth [15].
Animal studies reported that long-term use of high doses of fluoxetine decrease the fertility parameters in adult male rats [16–18]. Previous studies also noticed decreased weight of reproductive organs (testes, epididymides, ventral prostate) and slight decrease in body weight, when initial and final body weights were compared in the experimental groups [19].
Studies on rats showed that rats treated with 30 mg/kg/day of fluoxetine had decreased femur lengths compared to controls along with skeletal muscle degeneration, necrosis and regeneration. Inhibition of the SERT prevents bone formation & significant weight loss [20].
Goldstein BJ and Goodnik PJ reported that, in U.S. clinical trials for major depressive disorder, 11% of patients treated with fluoxetine reported anorexia (decreased appetite), 1.4% developed weight loss. Patients treated with 60mg of fluoxetine lost 0.45 kg weight in the 16 week double blind trial [21]. In the present study the weights of the Group 1 control rats kept on increasing with time, but the weight of the experimental rats continued to decrease with passage of time and on increasing the dosage of the drug. Beyazyuz M et al., observed significant reductions in the parameters of weight, total cholesterol and triglyceride after fluoxetine therapy in anxiety patients [22].
Michelson D observed 0.4kg of mean absolute body weight loss after 4weeks of fluoxetine therapy to depressive patients but after remission of depression weight gain occur. Weight gain was equal in patients on placebo and on fluoxetine respectively [23].
The FDA Pharmacologists Review of NDA also reported decreased body weight of mice treated with high dosage of fluoxetine. The same FDA review reported a one year oral toxicity study on Beagle dogs, received fluoxetine in dosages of 1mg, 4.5mg and 20 mg/kg/day for 6 months followed by a decrease in the high dose to 10 mg/kg/day for the next 6 months. Most of the high dose animals lost weight initially, but subsequently recovered [24]. In current study experimental rats who received high doses cannot be recovered subsequently.
Product information of fluoxetine hydrochloride oral solution by Eli Lilly and Co. reported that 30mg/kg oral fluoxetine hydrochloride in juvenile rats produces skeletal muscle necrosis and immaturity [25].
Byrd RA and Markham JK carried out a study on 344 pregnant Fischer rats and pregnant Dutch belted rabbits to see the developmental toxicology of fluoxetine hydrochloride. In rats, maternal toxicity was indicated at dose 12.5 mg/kg in the form of decreased body weight and decreased food consumption. Fetal viability, weight and morphology were not affected at any dose level. In rabbits, weight loss occurred at 2.5, 7.5 and 15 mg/kg. Food consumption was also decreased. Abortions and maternal mortality occurred secondary to anorexia and cachexia at dose of 15mg/kg of body weight. Fetal viability, weight and morphology were not affected at any dose level. Fluoxetine did not exhibit any toxicity toward the developing rat or rabbit conceptus at doses that were maternally toxic [26].
Landry M et al., investigated the effect of chronic fluoxetine treatment on 5-HT2A serotonin receptor-mediated neuroendocrine responses in young male rats. They treated these rats with saline or fluoxetine (10 mg/kg/day, I.P.) for 14 days and fluoxetine produced approximately 6% reduction in body weight. No change in ACTH, corticosterone and renin responses was seen. Oxytocin responses were approximately 50% attenuated in fluoxetine-treated rats, indicating a functional reduction in the 5-HT (2A) receptor-mediated oxytocin responses. Fluoxetine did not alter the plasma ACTH, corticosterone, or renin. According to them 5-HT2A serotonin receptors produce some neuroendocrine responses which were responsible for weight loss [11]. Weight loosing property of SSRIs can be used in obese psychiatric patients without any fear of withdrawal of drug.
Limitation
Limitation of current study is time and dose limitation that is 12 weeks was the maximum time of drug administration and the toxic dose was 40mg/kg/day. Hence, future studies can be conducted with increased duration of drug to ascertain its long term effect.
Conclusion
Present study concludes that SSRIs can cause weight change in the form of decrease of body weight. Although SSRIs (fluoxetine) causes weight loss but it is necessary to use them carefully when prescribing to a child, adolescent and pregnant lady.
(p < 0.01 - Highly significant, p < 0.05 – Significant, p > 0.05 – Non significant)