The early development of the human eye during the embryonic period and the appearance of different retinal layers have been studied [1–7]. A few authors have also studied differential expression of various proteins in the developing and adult human retina [8–10]. But our knowledge regarding the development of retinal layers remains partial; there is a need to unravel the different steps involved in the development, morphology and functioning of various elements which make the retina structural [7].
Details of in utero development of retina can provide significant insights, which may aid in formulation of better diagnostic criterion and prove helpful in development of curative treatment modules. Comprehensive accounts dealing with chronology and details of foetal development in all retinal layers are scarce in literature. Therefore, the present study was planned to record various histological changes occurring in different stages of foetal growth of central retina.
Materials and Methods
This is a cross-sectional histological study on central retina of 27 foetuses. All the specimens were studied after Haematoxylin & Eosin (H&E) staining. The study was conducted from April 2010 to December 2013.
This study was conducted on 27 normal foetuses received from both therapeutic and spontaneous abortions, preserved in 4% formaldehyde solution right after the delivery. Gestational age was determined, based on last menstrual period, confirmed by ultrasound examination and by crown rump length. The foetuses were divided into 12 groups for each Week of Gestation (WG) from 18th to 34th gestational week, with no representation from 23rd, 25th 27th, 29th and 33rd week of gestation. The foetuses with cranio-facial, chromosomal or neurological disorders were excluded from the study. Ethical clearance was taken from the institutional ethical committee.
Eye balls were enucleated with the help of an enucleation spoon. Each eye ball was sectioned parallel to the equator nearer to the posterior pole and fixed in 10% buffered formalin. This was followed by paraffin embedding, block making and sectioning. The sections were 5μ thick. The H&E staining was done by regular protocol. All specimens were examined under, light as well as fluorescent microscope.
Results
Foetal Retina
The adult human retina is arranged in ten distinct layers (from outside inwards). We studied their development in the central part of retina in 27 foetuses ranging from 18th WG to 34th WG [Table/Fig-1].
Tabular depiction of milestones in retinal development.
| Event | Week of gestation |
|---|
| Outer nuclear, outer plexiform, inner nuclear, inner plexiform and ganglion cell layers appear | 19 |
| Bruch’s membrane is formed | 20 |
| Photoreceptor layer differentiated with no migrating cells | 21 |
| Ganglion cell layer fully formed | 24 |
| Outer limiting membrane appear | 32 |
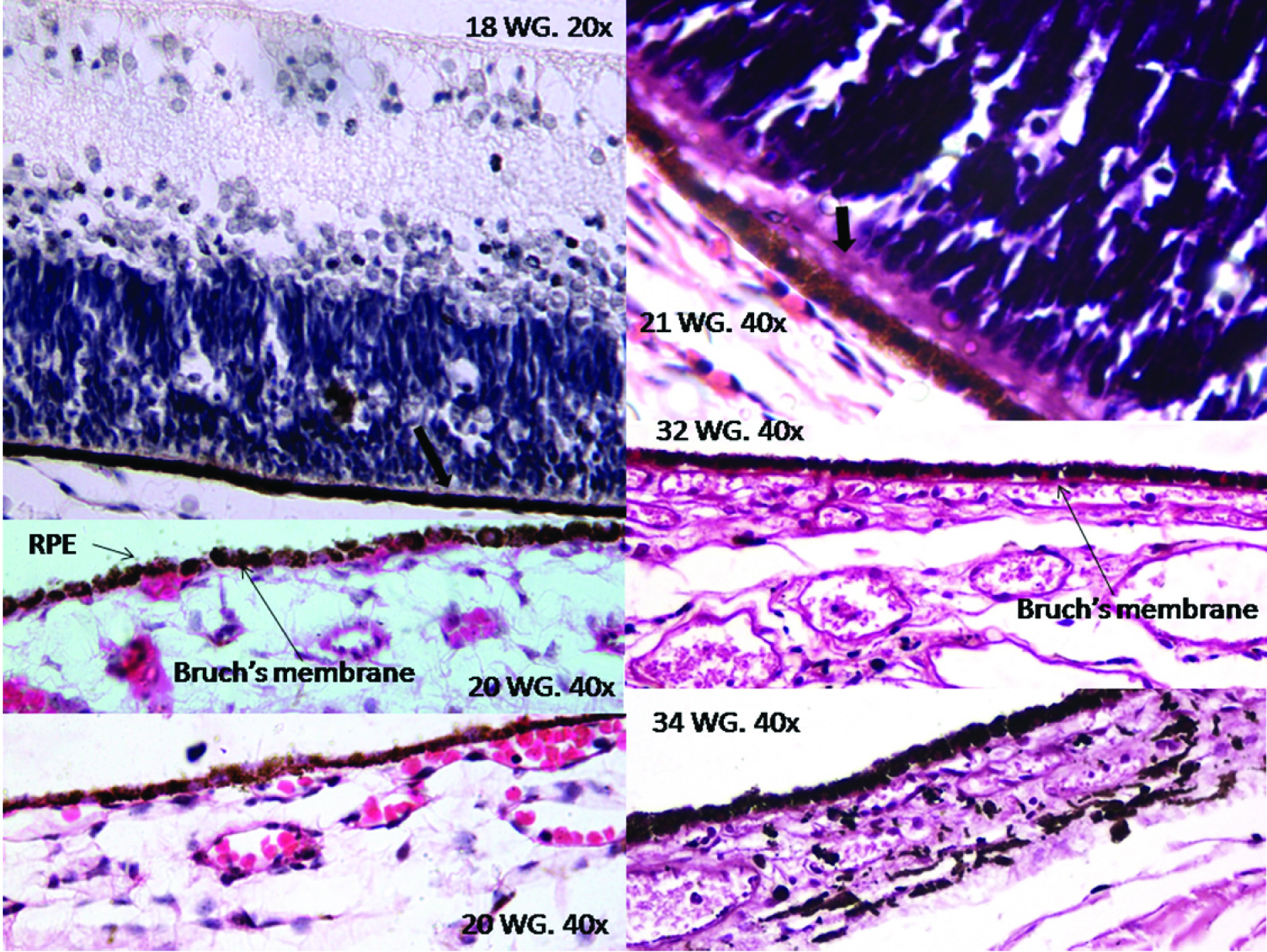
Retinal Pigment Epithelium (RPE): At 18th WG the retinal pigment epithelium was seen as a well-defined layer. The RPE was single cell thick with low cuboidal cells having central round nucleus. The cells of RPE were tightly adherent to each other. The cells were laden with discrete, dark brown to black, melanin granules. Melanin was present in all parts of the cells and was also covering the nucleus of the RPE cells. Apical cytoplasmic extensions into the photoreceptor layer were seen. Junction of the RPE and choroid was formed by basement membrane of the RPE cells only, with no contribution from the choroidal capillaries at this stage. In the retina obtained from the foetus of 19th WG similar features were noted. At the 20th WG the walls of the choroidal capillaries were seen abutting the basement membrane of the RPE cells. Hence, the proper Bruch’s membrane was formed at 20th WG. The apical cytoplasmic extensions into the photoreceptor layer became very prominent at 21st WG and could be seen as athick eosinophilic layer at the basal parts the photoreceptors. From 20th WG onwards fully formed RPE was seen [Table/Fig-2].
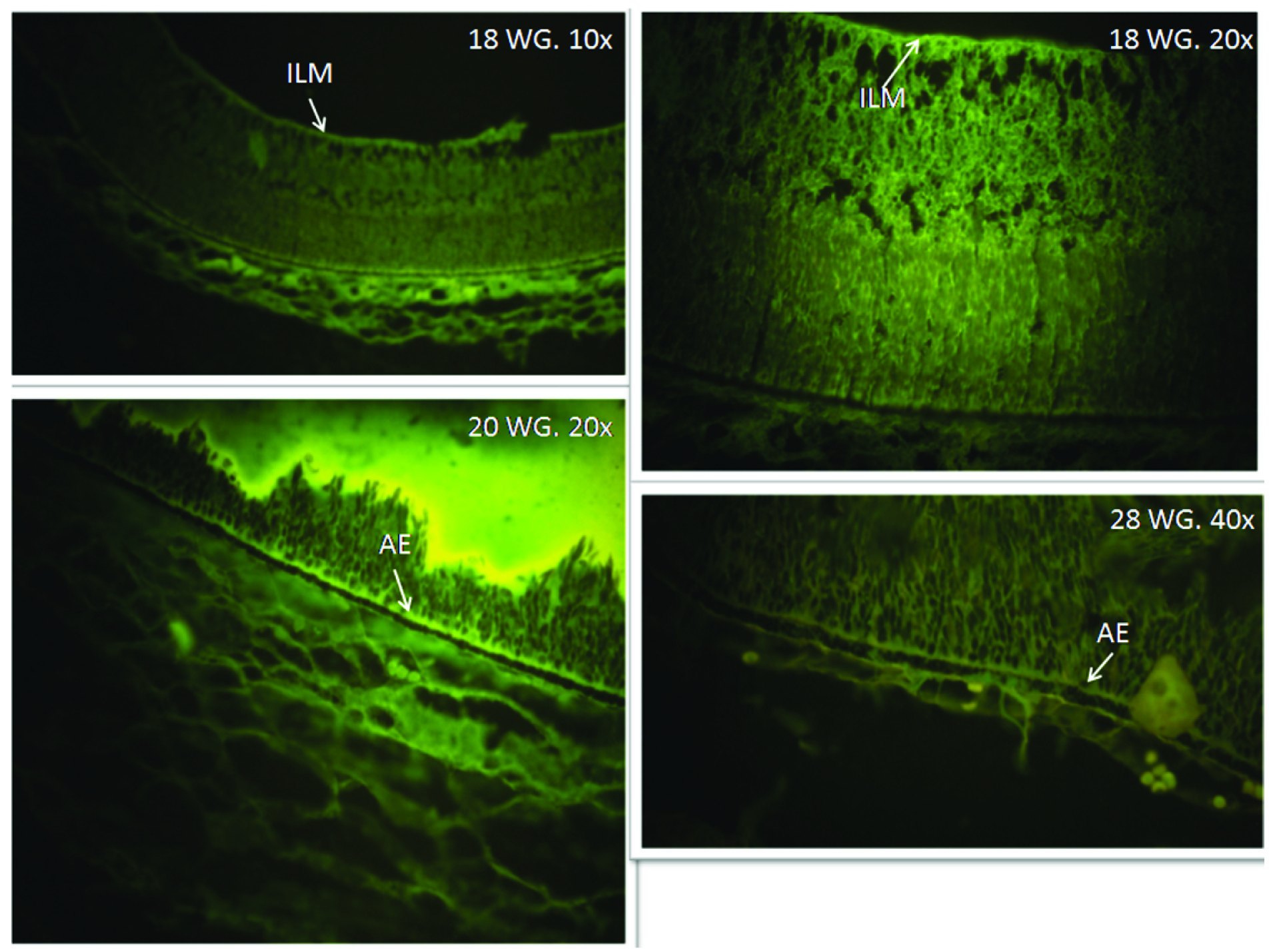
Lipofuscin and melanin are two pigment types present in the RPE cells. The lipofuscin is auto fluorescent pigment while melanin is not. We examined the H&E slides under the under fluorescent microscope using blue filter. Yellow fluorescence indicating the presence of lipofuscin was not detected in any of the specimens while melanin was seen as discrete non fluorescent granules present as clumps [Table/Fig-3].
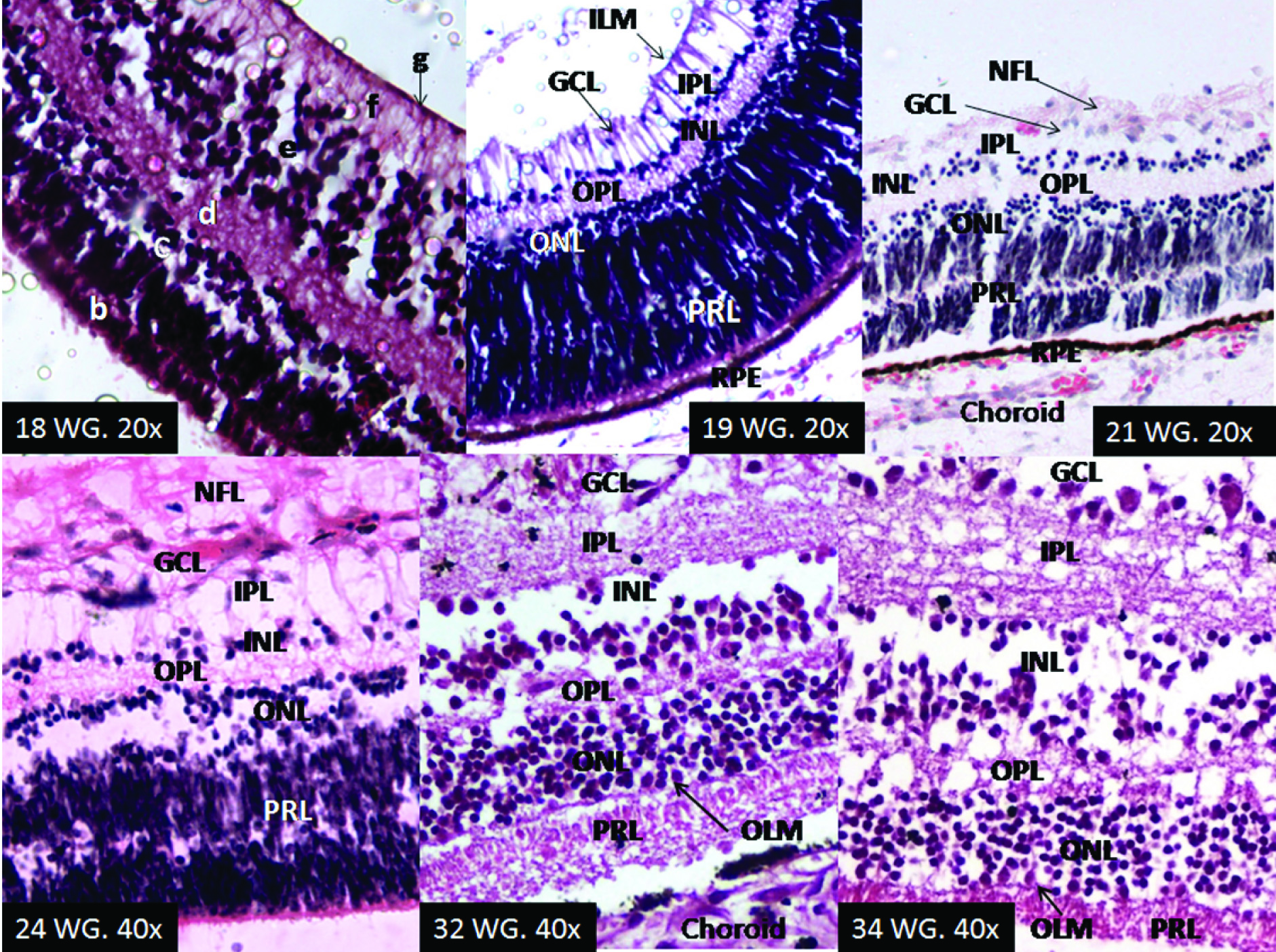
Layer of Rods and Cones (Photoreceptor): At the 18th week of gestation this layer appeared to be in the developing stage. As at this stage multiple nuclei could be seen at all levels scattered among the developing photoreceptors. These belonged to the cells which were in the process of migration towards the inner retinal layers. This layer was seen as the thickest layer. In one case it was more than half the thickness of the whole retina. At 20th WG this layer was more organized, in the form of longitudinal columns. From 21st WG onwards the photoreceptor layer appeared well defined, with very few other cells traversing them. The outer segments became more aligned at 28th WG [Table/Fig-4].
Outer Limiting Membrane (OLM): This membrane was not discernible from 18th through 28th WG. Though the junction of the photoreceptor and the outer nuclear layer appear as clear undulating line, no membrane was discernible till 28thWG. Probably because some cells were still in the process of migrating from the outer to the inner retinal layers. At 32nd week of gestation OLM is seen as a thin but distinct layer [Table/Fig-4].
Outer Nuclear Layer (ONL): At 18th WG the retina did not have ONL but had, what can be labelled as outer neuroblastic zone. Various types of nuclei could be seen in this zone. This was 7-8 cells thick. The zone was about half the thickness of the photoreceptor layer, but about quarter thicker than the inner neuroblasticzone. At 19th week ONL with all nuclei showing similar size and properties was discerned. This layer was formed by the nuclei of rods and cones. From 19th week onwards the proper outer nuclear layer was seen to be slightly thicker than the inner nuclear layer [Table/Fig-4].
Outer Plexiform Layer: At 18th week the neuropil or acellular layer was seen between outer and inner neuroblastic zones. This is formed by processes of migrating neurons. The neuropil was as thick as outer neuroblastic zone. Few cells could be seen migrating across it. The outer plexiform layer became evident at 19th WG with very few cells still crossing across it. From 20th week onwards this was seen as well-defined layer. This layer is formed by the processes of photoreceptor cells synapsing with the processes of horizontal, amacrine and bipolar cells [Table/Fig-4].
Inner Nuclear Layer: At 18th week of gestation inner neuroblastic zone with nuclei of variant features was seen. Some of the nuclei showed distinct characteristics of the ganglion cells nuclei which have differentiated but yet to migrate to the ganglion cell layer. From 19th to 21st WG inner nuclear layer became clear but it was very thin and still evolving. Few ganglion cells were still seen in this layer but in decreasing number. At 24th WG inner nuclear layer was thinner than outer nuclear layer while no ganglion cell was seen in this layer. This layer is composed by nuclei of the muller’s cells, horizontal cells, amacrine cells and bipolar cells [Table/Fig-4].
Inner Plexiform Layer (IPL): This layer was absent in retina of 18th WG. A layer of nerve fibres was seen beyond the inner nuclear zone at the 18th week of gestation, but as the fibres were linearly arranged, were connected to the ganglion cells and ended at the inner limiting membrane; this layer was labelled as the nerve fibre layer. IPL appeared at 19th WG where it was thicker than the outer plexiform layer but was similar in thickness as nerve fibre layer. Typical ribbons like synapses were seen in this layer from 20th WG onwards, which were formed by the processes of the amacrine, bipolar and ganglion cells [Table/Fig-4].
Ganglion Cell Layer (GCL): At the 18th WG the ganglion cells and their processes are clearly seen but the ganglion cells were admixed with the other nuclei present in the inner nuclear zone. The GCL first appeared as a distinct layer at 19th WG. This was single cell thick. The nuclei were largest in size among all the retinal cells. The nuclei were round, light staining with dispersed chromatin and distinct nucleoli. From 24th week of gestation onwards this layer appeared to be fully formed as the migration of the ganglion cells seemed to have completed by then [Table/Fig-4].
Nerve Fibre Layer (NFL): Well defined NFL was seen at 18th week. At this time the thickness of the NFL was about one fourth of that of inner neuroblastic zone. From 19th WG onwards the thickness increases and NFL was thicker than the IPL. This layer is formed by the efferent fibres of the ganglion cells which form the optic nerve [Table/Fig-4].
Inner Limiting Membrane: This was seen as a distinct thin eosinophilic membrane at 18th WG. This was formed by the basal lamina of muller’s cells [Table/Fig-4].
Depiction of the retinal pigment epithelium (RPE) and the Bruch’s membrane (BM) from 18th to 34thWG. At 18th WG the RPE was fully developed; with cuboidal cells laden with melanin pigment. Apical eosinophilic extension of the RPE cells up till the photoreceptor layer is clearly visible (thick arrow at 18th&21stWG). The partition between the choroid and the RPE was formed by the basement membrane of the RPE cells. At 20th WG distinct BM formed by the contribution of basement membrane of the RPE cells and abutting choroidal capillaries appeared. Appreciable increase in the thickness of the BM was found in the 32ndWG.
Retina seen under fluorescent microscope with green filter. The RPE was filled with non-fluorescent melanin pigment. Fluorescence indicating the lipofuscin granules within the RPE cells was not detected in any of the specimen. The apical extension (AE) of the RPE and inner limiting membrane (ILM) shows fluorescence.
Development of retinal layers. At 18th WG inner to the photoreceptor layer (b) (PRL) outer (c) and inner (e) neuroblastic zone were seen separated by the neuropil (d). The nerve fibre layer (f) and inner limiting membrane (g) (ILM) were clearly evident. At 19th WGthe outer nuclear layer (ONL) and the inner nuclear layer (INL) are differentiated, separated by the outer plexiform layer (OPL). The ganglion cells have started to migrate inwards from the INL to form the ganglion cell layer (GCL). The inner plexiform layer (IPL) was seen separating the INL from the GCL. At 21st WG the distinct GCL is seen. Some nuclei can be seen within the PRL; these are still in the process of migration to the inner retinal layers. Apical extension of the RPE cells into the base of PRL was seen as a thick eosinophilic layer at the bottom of the retina of 24 WG. Outer limiting membrane made it appearance at 32nd WG.
Discussion
Retina is a specialised nervous tissue which has exteriorized from the diencephalon. It has tissue equivalent of gray matter (retinal neurons), white matter (plexiform and nerve fibre layer) and glial cells (Müller cells and small astrocytes). The two layers of the optic cup differentiate: the cells of the outer layer produce pigment and eventually form the RPE. The cells of the inner layer of the optic cup constitute the neural retina. The pool of retinal progenitor cells expands by proliferation. The neural retina is composed of inner and outer neuroblastic zones, these subsequently generate the six types of neurons, i.e., retinal ganglion cells (RGCs), amacrine cells, horizontal cells, bipolar cells, and light-sensitive photoreceptor cells (rods and cones). In contrast to these neuronal cells, the Müller cells are glial cells [11]. These different cell types are generated from a pool of multipotent retinal progenitor cells (RPCs) in a sequence that is remarkably conserved across all vertebrates [12]. Bassett & Wallace found that during embryogenesis in the mouse, ganglion cells are generated first, followed by the production of cone photoreceptors, horizontal cells, and most of the amacrine neurons [13]. Bipolar neurons, Müller glia, the remaining amacrine neurons, and most rod photoreceptors are generated postnatally. However, they emphasized that there is considerable overlap in the production of retinal cell types at any given time.
We found that the apical microvilli of the RPE were seen at 19th week. Before the 20th WG the Bruch’s membrane was made up of basement membrane of the RPE only. At 20th WG proper Bruch’s membrane was formed with contribution from the choroidal capillaries. This would contribute to the formation of blood retinal barrier. It is evident that the RPE becomes fully functional at mid gestation. This is consistent with the observation by Nag and Wadhwa, that differentiation of most retinal neurons is attained by midgestation (20–21 WG) [6].
The scleral layers of of the outer neuroblastic zone give rise to the photoreceptors around 10–12WG; the cones at 10 and rods from 12, week of gestation onward [2,10,14]. According to other authors the photoreceptors develop around 15WG. Adult mosaic pattern is seen in the cone inner segments by 18–19 week [10,15]. Though the photoreceptors appear early, we found that they get better organized into longitudinal columns at 20th WG and are well defined at 21st WG. Outer limiting membrane became evident at 32nd WG. As this membrane develops from zonula adherences that attaches the ends of Müller’s cells to each other and to the rods and cones, it is probable that this layer evolves after inner segments of the photoreceptors are fully formed.
In our study at the 18th WG the outer neuroblastic and inner neuroblastic zones were separated by a neuropil. Outer neuroblastic zone contained various types of nuclei, including those meant for the inner retinal layers. Many nuclei could be seen migrating through the neuropil. Similarly, the inner neuroblastic zone contained several ganglion cell nuclei which were yet to migrate to form the separate ganglion cell layer. Hence, at 18th WG the cells were in the process of migration to establish all the distinct retinal layers. This is similar to the finding by Nag and Wadhwa, that at the 16-17 WG the inner nuclear layer was partly differentiated while the outer nuclear layer of photoreceptor cell bodies was just beginning to separate by the formation of the outer plexiform layer [8]. But they found prominent OPL at 16th WG and the distinct ganglion cell layer at 16-17 WG, while in our specimens these layers first appeared at 19th week of gestation. Some authors have reported earlier appearance of the IPL and the OPL; the IPL develops earlier (visible even before 15 WG) than the OPL, which is clearly seen only after 16 WG [2,16]. They are probably referring to the outer and inner neuropil.
The ganglion cells and their axons differentiate before 10WG in the central retina; ganglion cells lie together at this foetal age in the inner neuroblastic zone, from where they arise [1,2,10]. In our specimens single cell thick; distinct ganglion cell layer first appeared at 19th WG in the peripheral retina. We found a well differentiated nerve fibre layer at 18th WG. According to Hevner, connections to visual centres via theganglion cell axons are formed at 20th week [5]. By birth, all retinal layers and neurons are mature over its entire extent, except at the fovea, where photoreceptor differentiation (outer segments) continues up to 5 months postnatally [6]. The synapses are in a vitreal-to-scleral sequence in the primate retina [10,17].
Use of stem cells in retinal regeneration to treat various retinal disorders is being pursued actively and with positive results. Presently several phase I clinical trials for regenerative stem cell therapies are in progress. Many checks are needed before these therapies can translate into routine clinical practice. The monitoring of these developing RPE cells would require a thorough knowledge of developmental cues and timeline involved in retinal regeneration. [18,19]. This knowledge of timeline of embryonic eye development and base line data for normal devlopment of RPE can be used in future for ready reference to monitor the transplanted stem cells.
Conclusion
The period of mid gestation (19th-21st WG) seems to be the defining time for the retinal layers: The Bruch’s membrane was fully formed at 20th WG; the photoreceptor layer became well defined at 21st WG; both the nuclear layers and both the plexiform layers became distinct at 19th WG while outer limiting membrane was first appreciated at 32nd WG. Foetal retinal pigment epithelium was cuboidal and filled with melanin granules with no trace of lipofuscin pigment. We have attempted to provide the histological description of all the retinal layers across different stages of gestation. This data might aid in understanding the process of differentiation of ocular tissues in humans which is a prerequisite to make a better diagnosis for congenital retinal disorders and on a long term to improve therapy.