The diabetes epidemic is most pronounced in India. The International Diabetes Federation estimates the total number of diabetic patients to be around 62.4 million in India. This is further set to rise to 69.9 million by the year 2025. Diabetic patients have an increased cardiovascular risk [1,2]. This risk gets exaggerated by dyslipidaemia. Diabetic Dyslipidaemia (DD) is characterized by elevated fasting and post prandial plasma glucose along with increased triglyceride (TG), LDL-cholesterol (LDL-C) and decreased HDL-cholesterol (HDL-C) levels. Increased serum TG and low HDL-C often precede the onset of Type-2 diabetes mellitus (DM). In addition, LDL-C particles are converted to smaller, perhaps more atherogenic lipoproteins termed ‘small dense LDL-C’ (sd-LDL-C). These changes in lipid profile represent the major link between diabetes and the increased cardiovascular risk of diabetic patients [3,4].
Traditionally, diabetes and its accompanying dyslipidaemia are managed by a variety of permutations and combinations of oral anti-diabetic agents (ADAs) and hypolipidaemic drugs [5]. As far as dyslipidaemia is concerned, statins at best are able to benefit 20-30% patients only. Fibrates as well as Niacin have also not succeeded in bridging the therapeutic gap mainly due to the myotoxicity exerted by the former and lack of efficacy of the latter in all patients [6,7]. To address this gap in therapy the Peroxisome Proliferator-Activated Receptors (PPAR)-α/ γ agonists were developed. These molecules could correct both the dyslipidaemia as well as hyperglycaemia in DD. PPAR-α agonists (fenofibrate) and PPAR-γ agonist (pioglitazone) are approved for dyslipidaemia and type-2 DM. However, the latter and their use are fraught with problems such as fluid retention, weight gain and congestive cardiac failure [8].
Hence, the research focussed towards the development of dual PPAR-α/ γ agonist with an aim to control both lipid and glycaemic parameters with all the adequate safety profile. These dual agonists can help activate both PPAR-α and PPAR-γ receptors simultaneously. They help control, not only lipid but also glycaemic parameters and in addition, help reduce the risk of weight gain that is stimulated by PPAR-γ activation. This lack of weight gain was first observed with the use of fibrates that not only provided hypolipidaemic effects but also reduced body weight without affecting intake of food [9].
Saroglitazar is the first approved dual PPAR-α/ γ agonist for patients suffering from DD, which has shown efficacy in improving both, the lipid as well as the glycaemic parameters, with an excellent safety profile [10]. Saroglitazar was approved by Drug Controller General of India (DCGI) for launch in India in June 2013 [11]. Trails conducted so far in India have evaluated efficacy of saroglitazar on lipid profile and glycaemic parameters of patients with dyslipidaemia and DD. But none of the trials have evaluated the efficacy of add on therapy of saroglitazar to existing treatment regimen. Against this backdrop, the present study was designed to compare the effectiveness and safety of add on therapy of saroglitazar and fenofibrate with metformin in Indian patients with DD.
Materials and Methods
It was a prospective, randomized, open labeled, parallel group phase IV clinical trial. The study was approved by the Institutional Ethics Committee and conducted according to the ICMR guidelines for Biomedical Research on Human Subjects, 2006, and the Declaration of Helsinki. Subjects were recruited in the Department of Endocrinology, of a tertiary care teaching hospital and the study was conducted between August 2014 and February 2015. The study was registered under Clinical Trials Registry- India (Registration Number - CTRI/2014/10/005131).
Difference of means to be detected was set at 10%. Considering the true mean difference between two treatment groups as zero and the expected standard deviation of 10% in the study population, 80% power and α = 0.05, the number of patients required in each treatment group was 17. The sample size was calculated by using primer of biostatistics software (version 5.0).
Inclusion criteria were: Adults of either sex, aged between 18 and 70 and newly diagnosed cases of diabetic dyslipidaemia with plasma triglyceride level ≥ 150 mg/dl and HbA1C ≥ 6.5 and ≤8, were included in the study. None of the patients received any hypolipidaemic agent within last six months. All the patients were receiving only metformin 1000 mg per day.
Exclusion criteria were: Female patients who are pregnant or lactating, fasting plasma glucose (FPG) > 250mg/dl, post-prandial plasma glucose (PPPG) > 350mg/dl, LDL-C > 130 mg/dl, co-morbid cardiovascular, renal and psychiatric complications, co-administration of drugs that were likely to interact with saroglitazar, fenofibrate or metformin and that are likely to alter lipid profile and glycaemic status.
The primary end point of the trial was change in TG at week 12 visit. The secondary end points were changes in HbA1C, HDL-C, LDL-C and Total cholesterol (TC) at week 12 and FPG, PPPG at weeks 4, 8, 12 visits; body weight and incidence of adverse events (AE).
Each patient was evaluated for 12 weeks. Patients were initially screened clinically and biochemically on day 0 and at the follow up visits on weeks 4, 8 and 12. HbA1C and lipid profile estimations were made on day 0 and week 12. FPG, PPPG and body weight estimations were repeated at baseline and on all subsequent visits.
Included patients were randomly allocated in two different treatment groups using coin toss method for randomization. Patients included in group A received Metformin SR (Lupin diabetes care Ltd., Mumbai) 1000 mg/ day and fenofibrate (USV Limited, Mumbai) 160 mg/day. Similarly, patients included in group B received metformin 1000 mg/day and saroglitazar (Zydus discovery, Cadilla Healthcare Ltd., Ahemedabad) 4 mg/day. Study medications were dispensed thrice during the study period: First during baseline visit (study medication given for 4 weeks) and next during week 4 and 8 visit (each time for 4 weeks). Patients in group A were advised to take metformin 500 mg sustained release (SR) tablets orally twice daily after food and one fenofibrate (160 mg) tablet after breakfast. Patients in group B were advised to take metformin 500 mg SR tablets orally twice daily after food and one saroglitazar (4mg) tablet after breakfast for the 12 week study period. Compliance was assessed using the traditional pill count method at each follow-up visit. Patients with worsening clinical conditions or rising plasma glucose level were decided to be withdrawn prematurely from the study. All patients were advised to quit smoking and consumption of alcohol during the study period. Patients were monitored continuously throughout the study for any adverse event (AE). All AEs were reported as per the World Health Organization-Uppsala Monitoring Centre (WHO-UMC) criteria [12].
Statistical Analysis
Data analysis was as per modified intention to treat basis with patients reporting for at least one post-baseline follow-up visit being included in the analysis. Safety analysis was done for all enrolled patients. Categorical data in baseline demographic profile (gender) were analysed using Chi-square test. Numerical data were analysed by repeated measures ANOVA and paired t-test for intra group comparison and by unpaired t-test for inter-group comparison. Post hoc analysis was done by Tukey’s honestly significant difference (HSD) test. The p-value < 0.05 was considered to be statistically significant.
Results
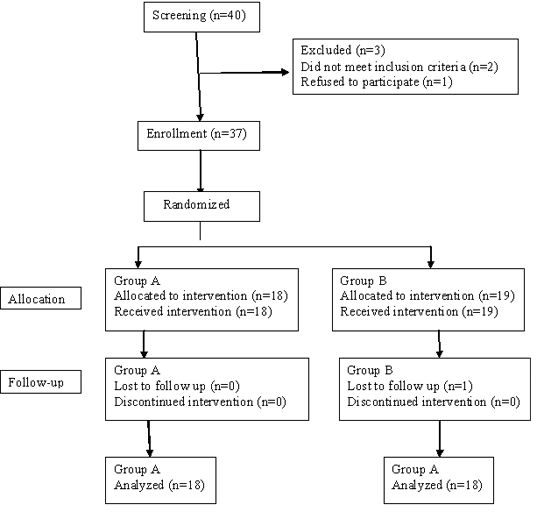
We encountered 40 patients who were duly screened. Of these 37 were enrolled in the study. Out of 37 patients, 18 patients were included in Group A (treated with metformin 1000 mg/ day and fenofibrate 160 mg/day) and 19 patients were included in Group B (treated with metformin 1000 mg/day and saroglitazar 4 mg/day). Of these 37 patients, 1 patient was lost due to follow up [Table/Fig-1]. Mean age of the patients was 58.1 and 62.6 years in Groups A and B respectively.
Results show that there is no statistically significant difference in baseline TG, HbA1C, FPG, PPPG, LDL-C and HDL-C levels among the study participants [Table/Fig-2]. After treatment, TG and HbA1C levels significantly decreased at week 12 from their respective baseline values (p< 0.05) in both groups [Table/Fig-3,4]. FPG and PPPG levels also significantly decreased at each of the follow up visits compared to their pretreatment values (p< 0.05) in both groups. On the flip side, LDL-C and HDL-C levels in both groups, did not show any statistically significant changes at week 12 from their respective baseline values.
Baseline triglyceride, glycosylated haemoglobin, fasting plasma glucose, postprandial plasma glucose, LDL- cholesterol and HDL-cholesterol levels (mean ± standard deviation) of groups A and B. No statistically significant difference among groups A and B in the baseline triglyceride, glycosylated haemoglobin, fasting plasma glucose, postprandial plasma glucose, LDL- cholesterol and HDL-cholesterol levels. Data were analysed by t-test.
| Parameters | Group A | Group B | p-value |
|---|
| TG (mg/dl) | 244.2 ± 30.6 | 245.9 ± 33.9 | 0.83 |
| HbA1C (%) | 7.1 ± 0.4 | 6.9 ± 0.6 | 0.22 |
| FPG(mg/dl) | 138.8 ± 5.7 | 136.9 ± 7.3 | 0.41 |
| PPPG(mg/dl) | 219.4 ± 15.5 | 220.1 ± 16.4 | 0.89 |
| LDL-C(mg/dl) | 114.1 ± 7.11 | 114.2 ± 10.76 | 0.96 |
| HDL-C(mg/dl) | 42.12 ± 5.19 | 40.18 ± 5.89 | 0.31 |
TG= Triglyceride, HbA1C = Glycosylated haemoglobin, FPG = Fasting plasma glucose, PPPG = Postprandial plasma glucose, LDL-C = LDL- cholesterol, HDL-C= HDL-cholesterol
Changes in triglyceride and HbA1C levels in Group A.
| Parameters | Week 0 | Week 12 | p-value |
|---|
| TG (mg/dl) | 244.2± 30.6 | 109.6 ± 5.56 | < 0.001 |
| HbA1C (%) | 7.1 ± 0.41 | 6.49 ± 0.53 | < 0.001 |
p < 0.001 at week 12 vs week 0. Statistical test used was paired t-test.
Changes in triglyceride and HbA1C levels in Group B.
| Parameters | Week 0 | Week 12 | p- value |
|---|
| TG (mg/dl) | 245.9 ± 33.91 | 98.44 ± 10 | < 0.001 |
| HbA1C (%) | 6.9 ± 0.56 | 5.6 ± 0.23 | < 0.001 |
P < 0.001 at week 12 vs week 0. Statistical test used was paired t-test.
When analysed between groups data shown at every follow up visit, add on therapy of saroglitazar with metformin significantly decreased FPG and PPPG levels, compared to add on therapy of fenofibrate with metformin [Table/Fig-5,6]. At the end of study, add on therapy of saroglitazar with metformin significantly decreased TG and HbA1C levels, compared to add on therapy of fenofibrate with metformin [Table/Fig-7]. Intergroup analysis, however failed to show statistically significant changes in body weight, LDL-C and HDL-C at the end of the study compared to baseline.
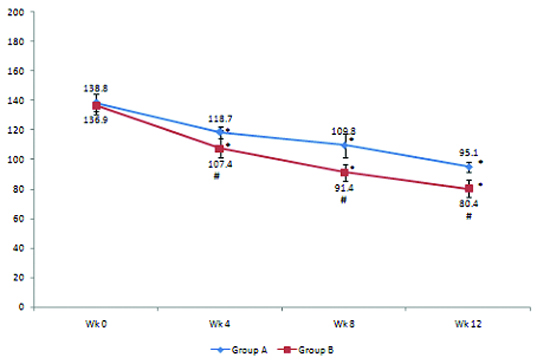
Changes in fasting plasma glucose (FPG) level (mg/dL) in groups A and B. FPG levels (mean± SD) in Group A are 118.7±4, 109.8±8.3, 95.1±3.1 at weeks 4,8, 12 respectively and in Group B are 107.4±12.4, 91.4±5.5, 80.4±6 at weeks 4,8, 12 respectively. * p< 0.05–Weeks 4, 8,12 versus week 0 in groups A and B. Statistical test used was repeated measures ANOVA followed by Tukey’s test. Intergroup comparison of FPG levels at weeks 4,8, and 12: At weeks 4, 8, and12- (#P < 0.05–Group B vs. Group A) . Statistical test used was t-test.
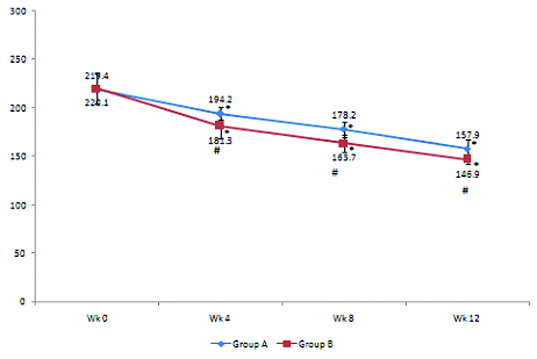
Changes in post-prandial plasma glucose (PPPG) level (mg/dL) in groups A and B. PPPG levels (mean± SD) in Group A are 194.2±4, 178.2±8.3, 157.9±3.1 at weeks 4,8, 12 respectively and in Group B are 181.3±12.6, 163.7±8.5, 146.9±4,8 at weeks 4,8, 12 respectively. * p < 0.05–Weeks 4, 8,12 versus week 0 in groups A and B. Statistical test used was repeated measures ANOVA followed by Tukey’s test. Intergroup comparison of FPG levels at weeks 4,8, and 12: At weeks 4, 8, and12- (#P < 0.05–Group B vs. Group A). Statistical test used was t-test.
Changes in triglycerides (TG) and glycosylated haemoglobin (HbA1C) level (%) in groups A and B at week 12. At week 12: P < 0.001–Group B vs Group A. Statistical test used was t-test.
| Parameters | Group A | Group B | p-value |
|---|
| TG (mg/dl) | 109.6± 5.56 | 98.4 ± 10 | < 0.001 |
| HbA1C (%) | 6.5 ±0.53 | 5.6 ±0.23 | < 0.001 |
Safety analysis was carried out for all randomized patients. We noted that two patients in group B complained of dyspepsia. Most AEs noted were during initial days of therapy and were mild in nature. None of these AEs lasted for over a week. None of the patients withdrew from the study due to AEs. Causality analysis showed they were in the “possible” category as per the WHO-UMC criteria.
Discussion
Incidence of dyslipidaemia is increasing worldwide at an alarming rate among people suffering type-2 DM. Despite availability of several oral ADAs and hypolipidaemic agents, current therapeutic strategies have limitations. Treatment of DD with predominant hypertriglyceridaemia is far from satisfactory. Effectiveness of conventional agents in treatment of hypertriglyceridaemia is inadequate and there is concern about safety. Hence, there is always need for newer therapeutic targets and newer drugs. Saroglitazar is a dual PPAR-α/ γ agonist, the first glitazar approved in the world and has emerged with a new hope to effectively treat DD with relative absence of AEs, especially with no increase of body weight [13,14].
Data from our study suggest, TG and HbA1C levels significantly decreased at week 12 from their respective baseline values (p< 0.05) in both groups. FPG and PPPG levels also significantly decreased at weeks 4, 8 and 12 compared to their pretreatment values (p< 0.05) in both groups. TG and HbA1C levels in group B decrease significantly compared to group A at week 12. FPG and PPPG levels in group B also decreased significantly compared to group A at every interval. Inter group analysis did not show any statistically significant change in body weight, LDL-C and HDL-C at week 12. However the duration of our study was only 12 weeks. There are few clinical trials which have evaluated efficacy and safety of saroglitazar in patients with DD. Recently conducted clinical trials PRESS V and PRESS VI have showed that saroglitazar 2mg and 4mg therapy has decreased triglycerides by 45%. These studies have also shown significant decrease of other atherogenic lipids like LDL-C, VLDL-cholesterol and TC [13–15]. In PRESS V study, saroglitazar decreased FPG and HbA1C levels significantly in a dose dependent manner and this decrease was similar to the efficacy of pioglitazone. Laboratory finding of safety parameters associated with saroglitazar were within normal limits and there was no significant alteration of body weights [14]. PRESS VI study evaluated saroglitazar in patients of DD inadequately controlled with statins. Results from PRESS VI study showed beneficial effects of saroglitazar on both lipid and glycaemic parameters [15]. Results of our study are also in agreement with these studies. In these trials saroglitazar emerged as the first drug having both lipid and glucose lowering effects and ensuring adequate control of DD with predominant hypertriglyceridaemia.
Safety analysis of our study shows an excellent safety profile with saroglitazar. Only two patients on saroglitazar reported to have dyspepsia. Dyspepsia was mild in nature, noted during initiation of therapy and lasted for about one week. None of the patients were withdrawn from the study. There was no reported case of serious life-threatening AE.
Limitation
Duration of the study was relatively short and the sample size was small due to logistic problems. These were the limitations of the study. We hope to Conduct longer studies to evaluate the efficacy and safety of this new and promising agent.
Conclusion
The results of this randomized, open labeled phase IV clinical trial showed that add on therapy of saroglitazar with metformin significantly decreased TG, HbA1C, FPG and PPPG levels compared to add on therapy of fenofibrate with metformin in Indian patients with DD. This add-on therapy of saroglitazar was also well tolerated.
TG= Triglyceride, HbA1C = Glycosylated haemoglobin, FPG = Fasting plasma glucose, PPPG = Postprandial plasma glucose, LDL-C = LDL- cholesterol, HDL-C= HDL-cholesterol
p < 0.001 at week 12 vs week 0. Statistical test used was paired t-test.
P < 0.001 at week 12 vs week 0. Statistical test used was paired t-test.