Angiogenesis is a fundamental process that affects physiologic reactions and pathological processes such as tumour development and metastasis [1]. Angiogenesis is the process of formation of new microvessel from the pre-existing vessels. It is thought to be balanced by angiogenic stimulators and angiogenic inhibitors which are inversely proportional to each other. These factors are thought to be released by tumour cells, stromal cells and inflammatory cells such as mast cells and macrophages [2].
Macrophages are versatile cells which participate in inflammatory reactions to foreign bodies and microbial invasion [3]. These are seen in various lesions like Peripheral Giant Cell Granulomas (PGCG), Central Giant Cell Granulomas (CGCG) and few others.
Peripheral and central giant cell lesions of the jaws are relatively uncommon benign reactive disorders, which arise either peripherally in periodontal ligament and mucoperiosteum of the alveolar ridge, or centrally in the bone. Both are virtually identical histologically, being characterized by the presence of numerous multinucleated giant cells (MGCs) and mononuclear cells (fibroblast and histiocyte-like cells and monocyte-macrophages) within a prominent fibrous stroma [Table/Fig-1,2] [4].
Although histologically similar, PGCG and CGCG differ clinically in terms of their behaviour. PGCG is considered as a reactive process which may arise in response to a local irritating factor that illustrates low recurrence after treatment once the irritating factor is eliminated. In contrast CGCG demonstrates an unpredictable clinical behaviour which can range from a slow asymptomatic growth without recurrence to rapid, painful and recurrent growth. Studies suggest these lesions are a part of spectrum of primary vascular proliferative mesenchymal lesions of the jaws [5].
Many studies have been addressed to determine the aggressiveness of these lesions considering various factors such as proliferative markers [6], osteotropic markers showing bone resorbing activity [7], MGCs [8] and angiogenic markers [5]. Studies have concentrated more on determining the role of MGCs by considering size, number of nuclei and distribution of giant cells. Very few studies have been done on angiogenic activity.
As a measure of angiogenic activity, most studies count the number of microvessels in tissue section expressed in the Microvessel Density (MVD). This technique was designated an easy prognostic indicator for clinical behaviour for a number of tumours [9].
Many markers have been used to observe angiogenesis and factors influencing it in various lesions. CD34 is one of the sensitive marker for vascular endothelium of both benign and neoplastic tissues as these molecules are found in association with endothelial microprocesses occurring at the tips of vascular sprouts suggesting that they play a role in cell adhesion and/ or migration. It has been speculated that CD34 is produced by endothelial cells and associated with angiogenesis [10]. CD68 is a macrophage marker and studies have reported its expression in giant cells [11].
We have previously conducted a study to prove the origin of giant cells as macrophages in CGCG and PGCG [12]. This study was conducted on the same samples with a purpose to correlate the impact of macrophages on angiogenesis in these two lesions by evaluating immunohistochemically MVD, Microvessel Perimeter (MVP) and Macrophage Index (MI).
Materials and Methods
This study involved paraffin embedded tissues of histopathologically diagnosed samples of CGCG and PGCG retrieved from archives of Department of Oral Pathology.
To evaluate the areas of angiogenesis and their relationship to macrophages a set of 20 cases of PGCG and 20 cases of CGCG were stained with CD34 the endothelial cell marker and CD68 commonly used macrophage marker by following the instructions provided by the manufacturer (BioGenex, USA).
Group I (n= 20): Clinically and histologically diagnosed cases of CGCG.
Group II (n=20): Clinically and histologically diagnosed cases of PGCG.
The immunohistochemical staining was done on 3μm thick tissue sections. In brief the sections were incubated at 55-60°C overnight before the day of staining followed by deparaffinization, rehydration and antigen retrieval.
All tissue sections were incubated in hydrogen peroxide to block endogenous peroxidase, washed in Tris Buffered Saline(TBS) and subjected to antigen retrieval using pressure cooker filled with citrate buffer solution (pH 6.0). Following this the sections were subjected to protein block and then treated with primary antibody for 60 minutes. Subsequently the sections were incubated with BIOGENEX SS Polymer for 30 minutes, washed in TBS before applying and also after applying SS polymer. Then the sections were treated with DAB chromogen, washed in water and counter stained with haematoxylin. Finally these sections were dehydrated, cleared and mounted with DPX.
Microvessel Density and Microvessel Perimeter Assessment
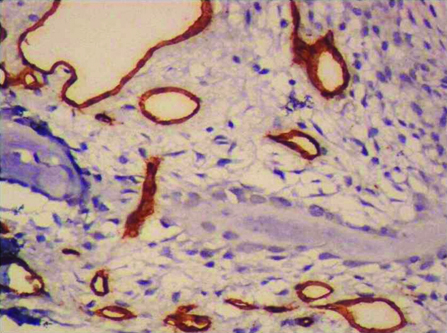
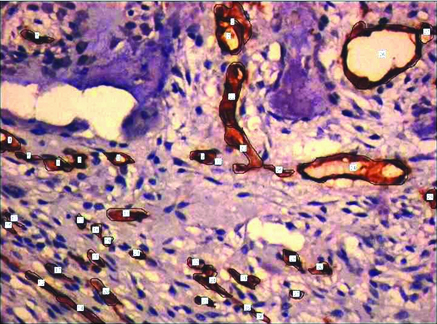
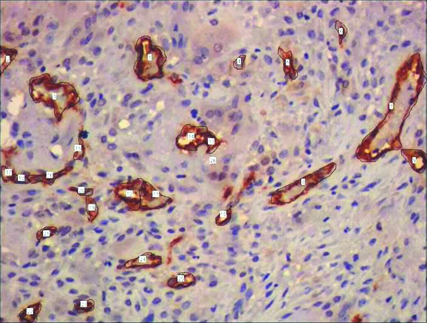
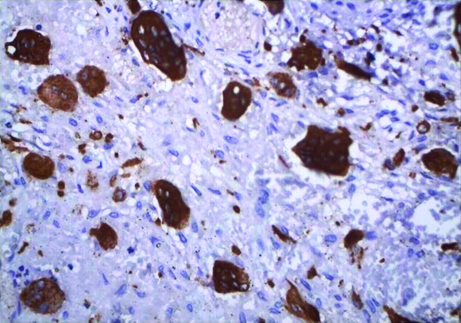
MVD and MVP were assessed by CD34 expression. All brown staining endothelial cell or endothelial cell cluster which could be clearly demarcated from tumour cells, adjoining microvessel and other connective tissue elements were considered for counting as a single microvessel [Table/Fig-3,4]. Vessel lumen, although usually present, were not necessary for a structure to be defined as a microvessel, red cells were not used to define a vessel lumen. Most vascularized areas within the lesions (hot spots) were chosen in low magnification (x10). Subsequently three non overlapping such fields were selected under high magnification (x40) for each slide. Images of these fields were captured using progress camera then imported to image analysis software (Image JAVA) this indicated the vascular tissue area. Then for each image total tissue area was also determined. Following this MVD was determined by calculating the ratio of vascular tissue area to total tissue area. MVP for each case was done by selecting three different fields under high power (x40) by tracing the outline of blood vessels. Mean of three such fields was recorded as MVP for each case [13] [Table/Fig-5,6].
CGCG showing CD34 stained blood vessels amidst bony trabaculae. (400x).
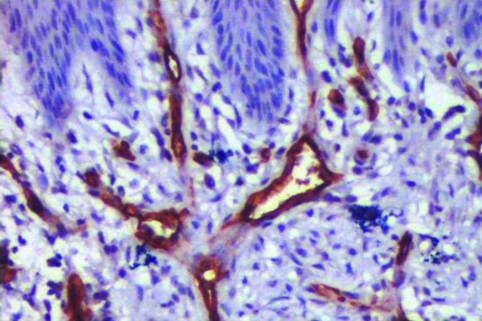
PGCG showing CD34 stained blood vessels with adjacent oral epithelium. (400x).
Representative photomicrographs of CGCG containing tracings of CD34 expression used for cytomorphometric measurements. (400x).
Representative photomicrographs of PGCG containing tracings of CD34 expression used for cytomorphometric measurements.(400x)
Macrophage index
The CD68 immunoreactivity was measured in the cells with a clearly defined immunostaining [Table/Fig-7,8]. These were assessed quantitatively by counting under light binocular microscope fitted with an eyepiece graticule which hauls a grid with 100 blocks which determine the perimeter of the selected field. Three such fields which had dense population of macrophages for each slide at a magnification of 40x were selected. The total numbers of macrophages along with all other cells within the given perimeter were counted. Later the total numbers of macrophages were divided by the total number of cells in each field which determined area percentage of each field. The mean of three such fields was taken as the marker expression estimation for each sample [7,8].
CGCG showing intense CD68 expression in scattered, irregularly shaped, numerous giant cell with few macrophages.(400x).
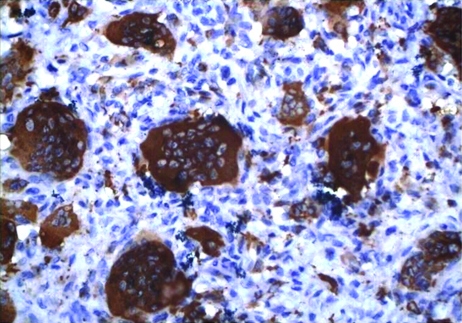
PGCG showing intense CD68 expression in scattered, irregularly shaped, numerous giant cell with few macrophages. (400x).
Statistical Analysis
Inferential statistical analysis was performed using Independent student t-test to assess the MVD, MVP and MI on continuous scale between Group I and Group II. Level of significance was determined at 5%. Further bivariate analysis using Pearson’s correlation test was carried out to see the relationship between MVD and MI in each group.
Statistical software
The statistical software namely Statistical Package for Social Sciences (SPSS) 22, IBM corp. was used for analysis of the data and Microsoft Word and Excel were used to generate tables.
Results
Micro vessel density
The endothelium was marked clearly by the brown CD34 immunostaining and variable size and shape blood vessels were identified in the stroma of both study specimens. Mean no. of MVD in group I and group II were 0.0042 and 0.0029 with a standard deviation of 0.0020 and 0.0013 respectively. An increase in MVD was seen in group I, the difference in the MVD between two groups was statistically significant with a p-value of 0.02 [Table/Fig-9].
Comparison of mean CD34 expression among the two groups using Independent student t-test.
| Groups | N | Mean | SD | Std. Error | Mean Diff | 95% CI for Diff | t | df | p-value |
|---|
| Lower | Upper |
|---|
| Group I | 20 | 0.0042 | 0.0020 | 0.00045 | 0.0012 | 0.00016 | 0.00237 | 2.334 | 38 | 0.02* |
| Group II | 20 | 0.0029 | 0.0013 | 0.00030 |
Statistically significant
Micro vessel perimeter
The mean MVP in group I and group II were 204.80 and 177.95 with a standard deviation of 40.19 and 38.56 respectively. It was found to be statistically significant among two groups with a p-value of 0.03 [Table/Fig-10].
Comparison of Perimeter among the two study groups using Independent student t test at p<0.05
| Groups | N | Mean | SD | Std. Error | Mean Diff | 95% CI for Diff | t | df | p-value |
|---|
| Lower | Upper |
|---|
| Group 1 | 20 | 204.80 | 40.19 | 8.99 | 26.85 | 1.64 | 52.06 | 2.156 | 38 | 0.03* |
| Group 2 | 20 | 177.95 | 38.56 | 8.62 |
Statistically significant
Macrophage Index
The CD68 positive macrophages were distributed within the fibro cellular stroma. Mean no of macrophages in group I and group II were 0.173 and 0.133 with a standard deviation of 0.052 and 0.029 respectively. It was found to be statistically significant with a p-value of 0.004 [Table/Fig-11].
Comparison of mean CD68 expression among the two study groups using Independent student t-test.
| Groups | N | Mean | SD | Std. Error | Mean Diff | 95% CI for Diff | t | df | p-value |
|---|
| Lower | Upper |
|---|
| Group 1 | 20 | 0.173 | 0.052 | 0.012 | 0.04 | 0.013 | 0.066 | 3.052 | 38 | 0.004* |
| Group 2 | 20 | 0.133 | 0.029 | 0.006 |
Statistically significant
The correlation between MVD and MI in Group I and Group II showed weak statistical significance with p-value of 0.387 in both groups and r-value of -0.205 and -2.92 respectively [Table/Fig-12,13].
Pearson correlation test to correlate the CD34 & CD68 expressions in Group I.
| Variables | Values | CD34 | CD68 |
|---|
| CD34 | r | 1 | -0.205 |
| p-value | | 0.387 |
| N | 20 | 20 |
| CD68 | r | -0.205 | 1 |
| p-value | 0.387 | |
| N | 20 | 20 |
Pearson correlation test to correlate the CD34 & CD68 expressions in Group II.
| Variables | Values | CD34 | CD68 |
|---|
| CD34 | r | 1 | -2.92 |
| p-value | | 0.387 |
| N | 20 | 20 |
| CD68 | r | -2.92 | 1 |
| p-value | 0.387 | |
| N | 20 | 20 |
Discussion
Angiogenesis is thought to be of crucial importance to the growth, maintenance and metastasis of solid tumours. It is multistep process involving the formation of new capillaries from an existing vascular network with remodeling of the extracellular matrix, endothelial cell migration and proliferation, capillary differentiation and anastomosis [14]. Angiogenesis is due to imbalance between pro and anti angiogenic factors produced from both normal and tumour cells.
Few factors which are known to stimulate angiogenesis are basic fibroblast growth factor, epidermal growth factor, interleukin 1, interleukin 2, transforming growth factor alpha, transforming growth factor beta, tumour necrosis factor alpha and vascular endothelial growth factor [1]. Among them vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) are thought to be the most potent inducers of angiogenesis [15].
It is now well established that vascularity and proliferative activity play key roles in tumour growth and invasiveness. Recently, it has been suggested that degree of tumour angiogenesis is related to clinical outcome, suggesting that angiogenic properties correlate with tumour aggressiveness [16]. MVD is usually considered one of the promising prognostic markers in several human tumours [17]. Several studies have been done to determine angiogenesis by using different vascular markers. Commonly used are VEGF, Von Willibrand Factor, CD31, and CD105. Shieh et al., have reported CD34 and CD31 were more sensitive than factor VIII-RA for evaluating the tumour blood vessel [18].
Several attempts have been made to understand the biological significance of angiogenesis and the factors affecting it in PGCGs and CGCGs and to establish histopathological parameters as reliable indicators of clinical behaviour [5].
CGCG is non neoplastic lesion which occurs in young individuals specially those under 30 years of age (60%). It is more common in mandible than maxilla with a ratio of 2:1 [19]. They arise more commonly in the anterior portion of jaws with mandibular lesions frequently crossing the midline [20]. Radiographicaly it presents as a destructive radiolucent area with either smooth or ragged border and sometimes with faint trabaculae. Along with these there may be displacement of teeth, expansion and perforation of cortical plates [19]. Histopathologically it comprises of few to many MGCs in a background of proliferative ovoid to spindle shaped mesenchymal cells and round monocyte-macrophages loose fibrillar connective tissue stroma. Areas of extravasated RBCs and haemosiderin deposition are often prominent. Foci of osteoid and newly formed bone are occasionally present [20].
PGCG is a reactive tumour like growth which presents as a red-blue nodular mass on gingiva or edentulous alveolar ridge. It most commonly develops in younger age group with a peak incidence being in 4th-6th decade of life. Frequency of mandibular lesion is more than maxillary lesion, commonly found in anterior region [19]. Radiographically it may show cupping resorption of the underlying alveolar bone. Histopathologically although similar to CGCG these lesions demonstrate surface epithelium which is separated from the underlying proliferative giant cell lesion proper by a zone of dense fibrous connective tissue [20]. The nature of giant cells in these lesions has been divisive for many years. R Rajendran suggested that giant cells may be phagocyte, foreign body, osteoclast, odontoclast or endothelial in origin [19].
A study on giant cell lesions by Peacock ZS et al., showed that the vascularity and level of angiogenesis in aggressive giant cell lesions were higher than those in non aggressive lesions [15].
A study by Susarla et al., showed that the staining density of CD34 was 2.5% more in aggressive giant cell lesions than non aggressive giant cell lesions [21]. Similar to these our study showed MVD was higher in CGCG compared to that of PGCG which was statistically significant (p= 0.02) thus suggesting CGCG as more aggressive lesion. In contrast O’ Malley et al., saw no differences for CD34 immunoreactivity between aggressive and non aggressive giant cell lesions [6]. Also, a study by Saulo G showed lesser number of CD105 positive and CD34 positive blood vessels in CGCG compared to that of PGCG [13].
Abbas et al., observed that the amount and dimension of blood vessel increase through normal epithelium to dysplastic epithelium and invasive carcinoma where it was greatest [22]. Similar study by Shivamallappa et al., observed that a statistically significant difference was noted between normal mucosa and carcinoma, leukoplakia and carcinoma but not between normal mucosa and leukoplakia with respect to microvessel perimeter [23]. Our study showed an similar increase in perimeter of blood vessels in CGCG than PGCG suggesting that it increases in aggressive lesions compared to non aggressive lesions.
The presences of macrophages in the stroma of CGCG and PGCG have been proved by several studies. In the present study the number of macrophages were significantly higher in CGCG than PGCG (p=0.004). A study by Leek et al., on breast carcinomas showed increased number of macrophages as a predictor of poor prognosis indicating it plays a role in belligerent behaviour of tumour [14]. Similarly Lu CF et al., correlated poor prognosis with a high frequency of macrophages in oral squamous cell carcinoma [24]. Similar to these in our study macrophages were higher in CGCG than PGCG hence we suggest that it may be considered as a predictor of poor prognosis signifying its aggressive behaviour.
Several studies have discovered the existence of correlation between angiogenesis and immune system comprising of mast cells, neutrophils, eosinophils, lymphocytes macrophages and others. In our study CD68 positive macrophages were significantly higher in CGCGs compared to PGCGs thus suggesting that macrophages play a role in angiogenesis because macrophages are key angiogenic effectors cells in the stroma. They produce a number of growth stimulators and inhibitors, proteolytic enzymes and cytokines that modulate the angiogenic process. The tumour associated macrophages derived conditioned medium can induce angiogenesis in various invivo model systems [25]. These findings suggest that angiogenesis may have a role in clinical behaviour that is why it is hypothesized that they play an important role in proliferation of granulomas.
Leek RD et al., indicated that macrophages play a role in angiogenesis and prognosis in breast cancers wherein he suggested that it could be due to the presence of more vessels, allowing the infiltration of more macrophages or the proangiogenic activity of macrophages inducing the proliferation, migration, and differentiation of endothelial cells [14]. A study by El-Rouby DH showed that increased tumour associated macrophages are associated with angiogenesis and higher histopathological grades in oral cancer [3].
Angiogenesis as well as the number of macrophages appeared to increase in CGCG in present study. These findings suggest that macrophages may up regulate the angiogenesis in these giant cell granulomas and angiogenesis do have a role in clinical behaviour. Inspite of all this we could not establish a positive correlation between MVD and macrophage index as the values were statistically insignificant. This insignificance may be presumed due to few samples taken for study.
Conclusion
The management of giant cell lesions of bone poses difficulty for maxillofacial surgeons and oncologists. These lesions have a high recurrence rate with locally aggressive and destructive nature inspite of which they are considered to be benign. Furthermore, there are no available biologic markers to predict clinical behaviour and its prognosis. In our study, we observed that MVD and macrophage index were elevated in CGCG compared to that of PGCG. Thus we hypothesize that MVD and macrophage have a role to play in their biological behaviour. However, we could not establish a positive correlation between MVD and macrophages as the inference could not be established based on smaller sample size taken. Hence we suggest undertaking study with larger sample size in order to clarify the mechanism operating in these granulomas and to provide appropriate therapeutic interventions.