Literature shows that 80% of patients undergoing surgery experience postoperative pain [1,2]. Majority (more than 80%) of these patients reported the intensity as moderate to severe [3]. Pain after surgery with several complications such as decreased wound healing, increased infections, prolonged hospital stays, readmission after discharge, increase morbidity and costs of hospital is a major challenge in postoperative care [1,4]. Therefore management of postsurgical pain plays a critical role in quality of the care plan [5–7]. Patients who experienced postoperative pain are exposed to decreased pulmonary function and the risk of developing thromboembolism due to immobility and suffer from nausea and vomiting. Also, cardiac workload, systemic vascular resistance, and myocardial oxygen consumption increase due to catecholamine release [8]. Laminectomy following lumbar herniation is one of the most common surgeries with an incidence of 10 to 40% in neurosurgery.
Annually, between 300,000 to 400,000 lumbar surgery was done. This statistics in the UK about 13,000 and over 250 000 in the US. In the United States alone $ 2.5 billion is spent for lumbar surgery. In addition annually, back pain causes loss of about 150 million days of work [9–11]. Postoperative pain, in these patients can continue for up to 3 days and 13-43% may become chronic [12]. The first choice for the management of postoperative pain is opioid. The use of opioids due to a number of adverse effects such as respiratory depression, nausea, vomiting, excessive sedation, dizziness, drowsiness, pruritus and urinary retention is limited [1,12–15].
To understand the importance of economic and physical aspects of postoperative pain, comprehensive studies on the efficacy of gabapentin in laminectomy patients, is necessary. The hypothesis of this study was that after laminectomy, a combination of celecoxib and gabapentin will demonstrate superior analgesic efficacy than either drug alone and cause fewer side effects.
To assess the comparative effect of gabapentin versus gabapentin plus celecoxib on pain and related complications after laminectomy.
Materials and Methods
This was an experimental study that was carried out at the Imam Khomeini Hospital affiliated with Ilam University of Medical Sciences, Ilam, IR, during the April - September 2015. The sample size was calculated according to information obtained from a pilot study with 10 patients and following formula.
n= (Z1+Z2)2 (2S2) /d2 = 38
Z1 = 95%= 1.96
Z 2 = 80%= 0.84 (test power)
S (an estimate of the standard deviation of VAS in the groups; 1.67 was obtained in a pilot study).
d (The minimum of the mean difference of VAS between the groups which showed a significant difference and was obtained 1.1.)
In a randomized, double-blind clinical trial, 114 patient American society of Anaesthesiologists (ASA) grade I or II, aged 20-60 years, scheduled for elective laminectomy under general anaesthesia were enrolled in this study. Patients were divided into three groups. In each group, there were 38 patients: group A (gabapentin) received 600 mg gabapentin 2 hours before surgery and 300 mg 6 hours after surgery, group B (gabapentin plus celecoxib) received 200 mg celecoxib plus 300mg gabapentin 2 hours before surgery and 6 hours after surgery and group C (placebo) received a placebo capsule orally 2 hours before surgery and 6 hours after surgery.
A simple random sampling design was used [Table/Fig-1]. Sampling with a sealed envelopes technique and coding was done. Coded as: code 1= gabapentin, code 2= gabapentin plus celecoxib, code 3= placebo. Coding and sealed envelopes technique was prepared by a nurse who was not participating in the study. The patients with drug abuse, history of allergic reaction to any of the study drugs, on non-steroidal anti-inflammatory analgesic, pregnancy, cardiovascular, metabolic, respiratory, peptic ulcer and renal failure, or coagulation abnormalities were excluded from the study. Neurosurgeon and anaesthesiologist were same in all patients. Anaesthesia was given with inj. Thiopentone (5 mg/ kg IV) and inj. Atracurium (0.5 mg/ kg IV), and maintained with Isoflurane (1-1.5%) and Nitrous Oxide (50%) in Oxygen. Fentanyl was given in the operation room according to patient need and clinical discretion. Patients were reversed with 0.05 mg/kg Neostigmine combined with 0.02mg/kg Atropine. Standard monitoring included electrocardiogram, noninvasive blood pressure, and pulse oximetry. We used the VAS to determine severity of pain. The pain severity was assessed 2, 4, 6, 8, 12 and 24 hours after surgery. The patient’s mean blood pressure (BP), heart rate (HR), respiratory rate (RR), saturation (SPO2), urine retention, vomiting, shivering, headache, dizziness, nausea, drowsiness, pruritus, discharge time and morphine consumption were recorded. Anxiety scores before surgery and patient’s satisfaction 24 hour after surgery were recorded.
CONSORT diagram of participant in the clinical trial.
Preoperative anxiety was assessed according to a seven-point scale (1 = relaxed, 2 = apprehension, 3 = mild anxiety, 4 = moderate anxiety, 5 = manifest anxiety, 6 = severe anxiety, 7 = very severe anxiety). The patient’s satisfaction with pain management was assessed on a 5-point scale; 0 = poor, 1 = fair average, 2 = moderate, 3 = good and 4 = excellent) during postoperative periods was recorded. Shivering was assessed on a scale with 0 = no shivering observed, 1 = shivering observed [1]. VAS rating is a standard tool for evaluating of pain severity having ratings from 0 to 10. 0 (0 means no pain and 10 means the maximum pain in this scale). To determine the validity of the Questionnaire, content validity was used. The questionnaire was given to 10 faculty members of Ilam University of Medical Sciences and was used after revision. To determine the reliability of questionnaire, Cronbach’s alpha test was used. The reliability of the questionnaire was 0.83. The study was approved by the Institutional Ethics Committee at the Ilam University of Medical Sciences, Ilam, IR, (EC: 94/H/269) and informed consent was obtained from all samples. This study was registered at the Iranian Registry of Clinical Trials (IRCT2015071222870N3).
Statistical Analysis
Collected data were analysed using the statistical software SPSS, Ver.16. (SPSS Inc, Chicago, IL, USA). Descriptive statistics, Chi-square test, one-way ANOVA, LSD and Tukey test and Repeated Measurement were performed to analyse the results. The p<0.05 was considered significant.
Results
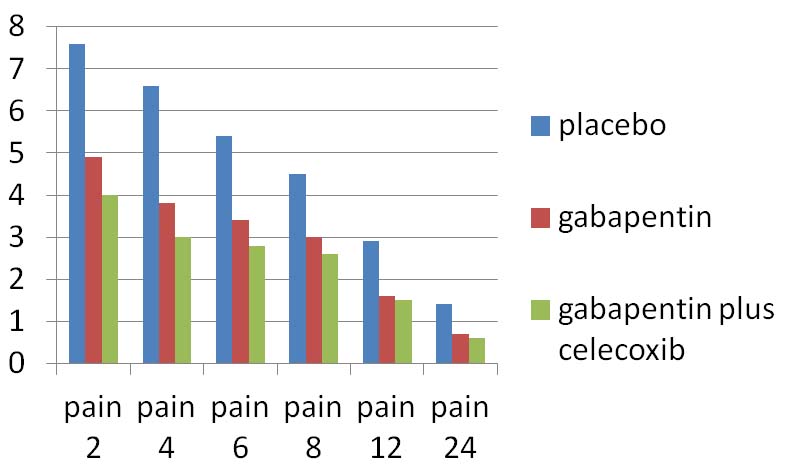
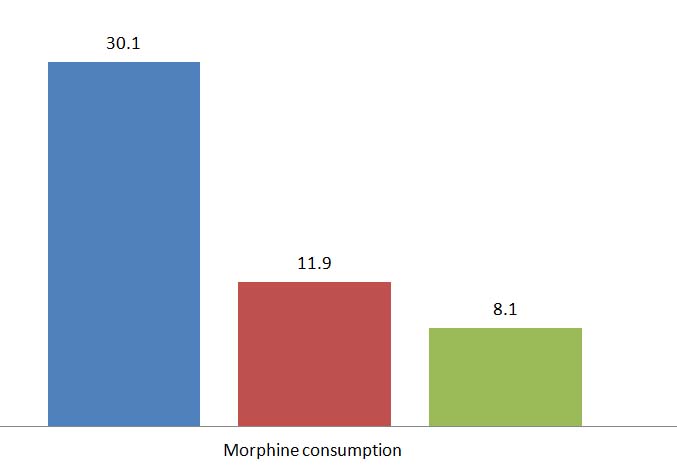
Baseline characteristics of the patients are shown in [Table/Fig-2]. Samples characteristics were not different among the groups (p > 0.5). One-way ANOVA test showed that the mean pain sevenity score and morphine consumption in the gabapentin plus celecoxib group were less compared to the placebo and gabapentin group respectively at various intervals (p < 0.001) [Table/Fig-3,4 and 5].
Patient characteristics, duration of anaesthesia and surgery.
| Characteristics | Placebo(n=38) | Gabapentin(n=38) | Gabapentin plus Celecoxib(n=38) | p-value |
|---|
| Age (year) [mean± sd] | 50.2±7.2 | 49.5±5.9 | 49.3±6 | >0.05 |
| Sex [(M/F) n%] | [28(73.7%), 10(26.3%)] | [31(81.6%), 7(18.4%)] | [25(65.8%), 13(34.3%)] | >0.05 |
| Married (n%) | 34 (89.5%) | 35 (92.1%) | 35(92.1%) | >0.05 |
| Mean duration of surgery ± SD (hr) | 2.11 ± 0.23 | 2.27 ± 0.28 | 2.17± 0.35 | >0.05 |
| Mean duration of anaesthesia ± SD (hr) | 2.35 ± 0.14 | 2.57 ± 0.18 | 2.49± 0.25 | >0.05 |
| BP (mm/Hg) | 132± 3.2 | 122±3.6 | 121±3.1 | >0.05 |
| PR (per/min) | 72.8± 3.4 | 73.6± 5.6 | 73.4± 5.4 | >0.05 |
| Spo2 | 95± 3.4 | 95± 2.2 | 96± 2.7 | >0.05 |
Severity of pain, Morphine consumption and Anxiety score in groups.
| Characteristic | Placebo(n=38) | Gabapentin(n=38) | Gabapentin plus Celecoxib(n=38) | p-value |
|---|
| Pain score by VAS at various intervals (hours) | M±SD | M±SD | M±SD | |
| 2 h after intervention | 7.6±1 | 4.9±0.7 | 4±0.7 | <0.001 |
| 4 h after intervention | 6.6± 0.9 | 3.8± 0.8 | 3± 0.9 | <0.001 |
| 6 h after intervention | 5.4± 0.5 | 3.4± 0.5 | 2.8± 0.7 | <0.001 |
| 8 h after intervention | 4.5±0.6 | 3± 0.8 | 2.6± 0.7 | <0.001 |
| 12 h after intervention | 2.9±0.7 | 1.6± 0.5 | 1.5±0.4 | <0.001 |
| 24 h after intervention | 1.4± 0.4 | 0.7± 0.3 | 0.6± 0.2 | <0.001 |
| Morphine consumption (mg) | 30.1 ± 0.6 | 11.9± 4.4 | 8.1± 2.7 | <0.001 |
| Anxiety score | 3.6± 0.7 | 2.4±0.5 | 2.5± 0.5 | <0.001 |
| Patient Satisfaction |
| Good | 4(10.5%) | 19 (50%) | 22 (57.8%) | <0.05 |
| Excellent | 0 (0) | 9 (23.6%) | 11 (28.9%) | <0.05 |
Severity of pain between groups.
Morphine consumption between groups.
According to LSD and Tukey test the mean pain severity in the gabapentin plus celecoxib group were significantly lower compared to the placebo and gabapentin group respectively at various intervals (p <0.001, p<0.05). In 8, 12 and 24 h after the intervention, although the mean pain severity in the gabapentin plus celecoxib group was lower than gabapentin group but there was no statistically significant differences between the two groups (p> 0.05) [Table/Fig-6].
Outcome of severity of pain between groups according to LSD and Tukey test.
| Outcome parameters | Between group | p-value |
|---|
| LSD | Tukey |
|---|
| 2 h after Intervention | Placebo | Gabapentin | <0.001 | <0.001 |
| Gabapentin plus Celecoxib | <0.001 | <0.001 |
| 2 h after intervention | Gabapentin plus Celecoxib | Gabapentin | <0.001 | <0.001 |
| Placebo | <0.001 | <0.001 |
| 4 h after intervention | Placebo | Gabapentin | <0.001 | <0.001 |
| Gabapentin plus Celecoxib | <0.001 | <0.001 |
| 4 h after intervention | Gabapentin plus Celecoxib | Gabapentin | <0.001 | <0.001 |
| Placebo | <0.001 | <0.001 |
| 6 h after intervention | Placebo | Gabapentin | <0.001 | <0.001 |
| Gabapentin plus Celecoxib | <0.001 | <0.001 |
| 6 h after intervention | Gabapentin plus Celecoxib | Gabapentin | <0.001 | <0.001 |
| Placebo | <0.001 | <0.001 |
| 8 h after intervention | Placebo | Gabapentin | <0.001 | <0.001 |
| Gabapentin plus Celecoxib | <0.001 | <0.001 |
| 8 h after intervention | Gabapentin plus Celecoxib | Gabapentin | >0.05 | >0.05 |
| Placebo | <0.001 | <0.001 |
| 12 h after intervention | Placebo | Gabapentin | <0.001 | <0.001 |
| Gabapentin plus Celecoxib | <0.001 | <0.001 |
| 12 h after intervention | Gabapentin plus Celecoxib | Gabapentin | >0.05 | >0.05 |
| Placebo | <0.001 | <0.001 |
| 24 h after ntervention | Placebo | Gabapentin | <0.001 | <0.001 |
| Gabapentin plus Celecoxib | <0.001 | <0.001 |
| 24 h after intervention | Gabapentin plus Celecoxib | Gabapentin | >0.05 | >0.05 |
| Placebo | <0.001 | <0.001 |
Repeated measurement analysis showed that the mean pain score in the gabapentin group (p< 0.001), placebo group (p< 0.05) and gabapentin plus celecoxib group (p<0.001) were significantly different in various intervals.
The mean anxiety score, shivering, nausea, vomiting and pruritus in the gabapentin group were significantly lower compared to the placebo and gabapentin plus celecoxib group respectively (p < 0.001, p<0.05) [Table/Fig-3,7].
Frequencies of adverse effects between groups.
| Characteristic | Placebo(n%) | Gabapentin(n%) | Gabapentin plus Celecoxib(n%) | p-value |
|---|
| Vomiting | 15 (39.5%) | 4 (10.3%) | 6 (15.8%) | <0.05 |
| Shivering | 16 (42.1%) | 4 (10.5%) | 6 (15.8%) | <0.05 |
| Headache | 4 (10.5%) | 5 (13.2%) | 6 (15.8%) | 0.792 |
| Dizziness | 8 (21.1%) | 13 (34.2%) | 13 (34.2%) | 0.371 |
| Nausea | 17 (44.7%) | 5 (12.8%) | 11 (28.9%) | <0.05 |
| Drowsiness | 2 (5.3%) | 16 (42.1%) | 13 (34.2%) | <0.001 |
| Pruritus | 17 (44.7%) | 7 (18.4%) | 8 (21.1%) | <0.05 |
| Urine Retention | 9 (23.7%) | 5 (13.1%) | 6 (15.8%) | 0.431 |
The frequencies of drowsiness (42.1%) in the gabapentin group were significantly high compared to the placebo and gabapentin plus celecoxib group respectively (p<0.001, p< 0.05). No statistically significant difference was observed between the groups in relation to headache, urine retention and dizziness (p> 0.05) [Table/Fig-7]. In the gabapentin plus celecoxib group patient satisfaction was significantly higher compare to the placebo and gabapentin group (p< 0.05) [Table/Fig-3].
Discussion
Postoperative pain, shivering, nausea, vomiting and urine retention are common complication impairing the quality of postoperative recovery, leading to postdischarge readmissions, causing chronic pain after surgery and increasing morbidity and costs [3]. In the past decade, despite our knowledge of pain physiology approximately 80% of patients undergoing surgical procedures experience postoperative pain [18]. Although opioids are the first choice for postoperative pain management they are associated with side effects [19]. Therefore, the multimodal analgesic approach has been recommended as an alternative treatment for the management of postoperative pain [18].
Treatment of postoperative pain and complications such as shivering, nausea and vomiting is a common challenge in postoperative care process and the basic principle in the early mobilization and well-being of the surgical patient [7]. Our results showed that gabapentin with celecoxib significantly reduced pain, overall morphine consumption but preoperative anxiety, pruritus, postoperative shivering, nausea and vomiting were less in the gabapentin alone group. In the gabapentin with celecoxib group patient satisfaction was significantly higher compared to the placebo and gabapentin group. This finding was consistent with the previous studies of the effects of gabapentin on postoperative pain and complications [20–23].
Reuben et al., concluded following laminectomy the pregabalin/celecoxib combination was the most effective treatment for reducing pain and patients in these groups had fewer postoperative complications of nausea and vomiting [18]. Syal et al., found that in patients after open cholecystectomy Acetaminphen 1000 mg plus 1200 mg Gabapentin is effective on pain and morphine consumption but increase prevalence of nausea, vomiting and sedation was seen [24]. Ozgencil et al., in a trial of 90 patients after laminectomy found in the gabapentin and pregabalin groups, preoperative anxiety, pruritus, overall morphine consumption, postoperative shivering were significantly lower and patient satisfaction was significantly higher than those in the placebo group [1]. Kumar et al., found that pregabalin after laminectomy significantly decreases pain score compared to the placebo, but the effect was less than to tramadol group. The need for rescue analgesia was less in tramadol group. In pregabalin group the postoperative complications such as nausea, vomiting, and drowsiness were less in comparison to tramadol [8]. Gianesello et al., showed that in spinal surgery pregabalin significantly decreases VAS scores, postoperative incidence of constipation and nausea/vomiting than in the placebo group [12]. Liporaci Juniot in spinal surgery found that gabapentin significantly reduced the pain intensity after surgery [25]. Rahimi et al., concluded that gabapentin 800 mg daily can decreases the pain severity after laminectomy than those in the placebo group and received gabapentin 400 mg daily [26]. Pandey et al., concluded that prophylactic gabapentin in laparoscopic cholecystectomy patients significantly decreases nausea and vomiting and fentanyl consumption [27]. Bafna et al., in patients undergoing elective gynecological surgeries under spinal anaesthesia found that preemptive use of gabapentin and pregabalin significantly reduces the postoperative rescue analgesic requirement and increases the duration of postoperative analgesia [28].
Studies emphasized on the use of nonopioid analgesic drugs and multimodal therapy for preventing pain in the preoperative period. Several benefits of multimodal therapy include improved pain relief, reduction in preoperative stress response, shorter hospital stays, decreased hospital costs, improved patient satisfaction, and a reduction in postoperative morbidity and mortality [12]. The sedation levels in the gabapentin group were significantly higher compare to the placebo and celecoxib group. This finding suggests that gabapentin can raise the sedation levels of patients [1].
Gabapentin, a structural analog of gamma amino butyric acid is an antiepileptic drug that has demonstrated analgesic effect in diabetic neuropathy, post-herpetic neuralgia and neuropathic pain [27,29]. The mechanism of action of gabapentin by binding to the α2δ subunit of voltage dependent calcium channels [30]. Gabapentin has antiallodynic and antihyperalgesic properties that decrease the hyperexcitability of dorsal horn neurons induced by tissue injury. Decrease in central sensitization by an antihyperalgesic drug such as gabapentin can reduce acute postoperative pain. Gabapentin may also prevent opioid tolerance [31]. The anxiolytic effects of gabapentin have been reported in previous study [1]. On the other hand the effect of gabapentin has been reported in the treatment of nausea and vomiting in patients receiving cytotoxic drugs and patients under laparoscopic cholecystectomy. The mechanism of gabapentin in the prevention of emesis due to decreases of tachykinin neurotransmitter activity has been postulated to be useful [27]. The anticonvulsive, anxiolytic and analgesic effects of gabapentin can reduce the incidence of shivering [1].
Recently, celecoxib has been demonstrated to have analgesic efficacy after spinal surgery [32,33]. COX-2 NSAIDs have been demonstrated to have analgesic efficacy during pain at rest and with movement [34]. Meta-analysis studies highlighted the importance using of NSAIDs and COX-2 inhibitors in the multimodal analgesic approach [35]. The cardiovascular side effects of COX-2 inhibitors are widely accepted. Several studies of longterm use of selective COX-2 inhibitors shows an increased risk of incident atrial fibrillation, myocardial infarction, stroke, and heart failure. The limited use of selective COX-2 inhibitors is due to these side effects [36–41].
Limitation
The limitations of our study include relatively small sample size and the subjective perception of pain by patients. All patients enrolled in this study were operated upon by a single surgeon and data collected from a single center that was the strength of this study.
Conclusion
To conclude, our findings revealed that after laminectomy, 200 mg celecoxib plus 300mg gabapentin twice a day is the alternative in multimodal analgesia. The adverse effects of gabapentin alone were found to be less. It also decreases the amount of morphine consumption for postoperative pain management as well as increased patient satisfaction in gabapentin plus celecoxib group.