Rheumatoid arthritis (RA) is a major public health problem worldwide and indeed, associated with excess cardiovascular morbidity and mortality [1,2]. In particular, arthritic patients have at greater risk for manifestation of cardiovascular disease (CVD), including myocardial infarction and death [3]. Although precise etiology of this debilitating disease is poorly understood, probability of RA patients to develop future CVD risk appears to be more due to involvement of some common conventional CVD risk factors such as high body mass index, dyslipidaemia, smoking and genetic factor [4–6]. Increased TC/ HDL-C ratio i.e. atherogenic index, is also an important prognostic marker for cardiovascular disease and the risk of myocardial infarction increases considerably when this ratio is higher than five [7]. In addition, the increased prevalence of CVD in RA patients has renewed the interest of researchers to identify non-traditional risk factors of CVD in this disease.
The non-traditional risk factors such as systemic inflammation and markers of oxidative stress have been implicated in the increased CVD burden of arthritic patients [7]. To elucidate the relationship between chronic inflammation and reactive oxygen species (ROS) content in tissues, the role of inflammatory cytokines such as Interleukin 6 (IL-6) and Tumour Necrosis Factor - α (TNF-α), and marker of systemic inflammation such as C- reactive protein (CRP) in RA as well as in CVD, cannot be neglected [8–10]. Moreover, continued active inflammation exerts its cellular side effects mainly through excessive production of ROS [11]. These ROS confer their toxic effects through cascade of deleterious events such as DNA strand breakage, damage to endothelium, cartilage, structural proteins and oxidation of LDL, i.e. lipid peroxidation, which eventually lead not only to RA progression but also development of CVD risk in arthritic patients as well [10,12,13].
Antioxidant defense system plays a crucial role in scavenging ROS by incorporating antioxidant enzymes and antioxidants. In this context, paraoxonase (PON), a calcium-dependent A-esterase, synthesized primarily in the liver, secreted into the serum as HDL-associated enzyme and execute anti-atherogenic property of HDL, has received much attention in various diseases associated with CVD complications [14,15]. In addition, alteration in total antioxidant activity (TAA) and increased levels of lipid peroxides induces the loss of homeostasis in vascular system that eventually elaborates the degeneration of vascular endothelium in arthritic patients and increases the CVD risk [9]. In previous studies, the association of altered plasma PON activity and increased incidence of CVD are well documented [7,15]. These observations direct the need and importance of plasma PON assessment in RA patients, to predict the risk of CVD, which have yet not been clearly elucidated.
Therefore, the aim of present study was to assess plasma PON activity in RA patients, as one of the important non-traditional therapeutic target and co-relate it with markers of systemic inflammation, oxidative stress and other variables (Disease activity score-28 and Erythrocyte sedimentation rate) of RA, in order to determine their probability to predict CVD risk in North Indian RA patients.
Materials and Methods
The present study was case control study. In a period of 8 months (February to October 2011), 118 patients visited to outpatient clinic of the hospital with joint complaint were screened for RA. A total of 40 RA patients fulfilled the inclusion criteria, were recruited in the study as patient group, 57 patients did not meet the inclusion criteria and 21 subjects refused to participate.
Inclusion criteria: Criteria recommended by the American Rheumatism Association were used for the diagnosis of RA [16]. The study protocol was approved by Institutional Ethics Committee. Objective oriented information including demographic information, family history of CVD, RA or both and limited physical examination was completed on all the subjects after taking the informed consent. The number of swollen and tender joints (28 joint count) and patient’s assessment of pain on Visual Analog Score (VAS) were registered. Disease activity score-28 (DAS28) were calculated using erythrocyte sedimentation rate [17]. The level of RA disease activity was interpreted as low (DAS28 ≤ 3.2), moderate (3.2 < DAS28 ≤ 5.1) and severe disease activity (DAS28 > 5.1). Height and weight were measured with subject barefoot and lightly dressed. The Body Mass Index (B.M.I.) was calculated as {B.M.I. = Weight (kg) / Height (metre2)}.
To diminish any confounders developed by other arthritic complications, 40 RA patients with positive rheumatoid factor were recruited and their disease duration was recorded. The sample sizes were calculated by statistician of the college keeping principle, administrative issues and cost in mind [18]. Among the 40 RA patients, 12 RA patients (9 males and 3 females) had family history of arthritis and hypertension. In addition, out of 40 RA patients, 23 patients were under medical treatment including supplementation of antioxidants and non-steroidal anti–inflammatory drugs. They were also not excluded from the study because no supplements or analgesic drug would be taken by them in the seven days before entry into the study. However, there was no restriction or withdrawal on the conventional anti-rheumatoid drugs treatment.
Exclusion criteria: None of the patients had family history of concomitant diseases, such as diabetes mellitus, hepatitis, renal failure and neurological disorder. In addition, patients with established cardiovascular complications, pregnancy, lactation, obesity (B.M.I > 25), hypertension (B.P. >120/80 mmHg), smoking habit, renal failure, liver disease, hypothyroidism or who did not follow study instructions were also excluded from the study.
Forty age and sex matched healthy individuals (20 males, 20 females) from college staff and their family members were recruited as controls after taking their informed consent. They had neither joint complaints nor any rheumatological disease. Blood samples (approximately 10 ml) were collected in sterile EDTA vials by venous puncture from overnight fasting subjects. Plasma was separated by centrifugation of blood at 1000 g for 15 min at room temperature and stored at -800C until use. Synovial fluid (1 ml), aspirated form the knee of patients by needle aspiration with the use of a sterile technique, was placed in a sterile polypropylene tube and centrifuged at 1000 g for 10 min at room temperature to remove cells and joint debris, then frozen at -800C until analysis. The ESR levels were determined according to the Westergren method. Plasma CRP, synovial IL-6, markers of oxidative stress, i.e. erythrocyte lipid peroxidation and plasma TAA levels and PON activity were estimated in controls as well as in RA subjects.
Plasma CRP and synovial IL-6 levels were measured using commercially available ELISA kits (R&D Systems, USA), according to manufacturer’s instructions. Plasma lipid profile contents (Total Cholesterol, Triglycerides and HDL cholesterol) were analysed enzymatically using kit obtained from (Randox Laboratories Limited, Crumlin, UK). LDL-cholesterol levels were calculated by Friedwald’s formula [19]. LDL-C = TC – {(TG/5) + HDL-C}
Erythrocyte malondialdehyde (MDA) levels were measured as thiobarbituric acid reactive substances, after preparation of haemolysate. In this method, the heat induced reaction of malondialdehyde (MDA) with Thio Barbituric Acid (TBA) in the acid solution forms a trimethine colored substance, which was measured spectrophotometrically at 532 nm [20].
Plasma total antioxidant activity was estimated spectrophotometricaly by the method involving reaction of standardized solution of iron EDTA complex with hydrogen peroxide, i.e. Fenton type reaction, leading to the formation of hydroxyl radicals. This reactive oxygen species degrades benzoate, resulting in the release of TBARS. Antioxidants from the added plasma cause the suppression of production of TBARS. The reaction was measured spectrophotometrically at 532 nm [21].
Plasma PON activity was estimated by using p-nitrophenyl acetate (5.5 mM/L) as a substrate [22]. The increase in the absorbance of formed p-nitrophenol was measured at 412 nm spectrophtometrically. The activity of plasma PON was measured in Tris buffer (20 mM/L; pH 8.0) containing 1mM CaCl2. The generated product p-nitrophenol was calculated by using molar extinction coefficient of 17000 per mole/cm at pH 8.0. Results were expressed as Units/ml (1 nmol p-nitrophenol formed per minute).
Statistical Analysis
The data collected from patients and control were entered separately in Microsoft Excel sheet of windows 2007 and values were expressed as Mean±SD. The significance of mean difference between patient and control groups was compared by using Student’s t-test. The distribution of ‘t’- probability was calculated depending on ‘n’ and significance of test was obtained. p-value <0.05 and <0.001 were considered as significant and highly significant, respectively. p-value <0.1 was considered as insignificant. In addition, correlation analysis between aforesaid parameters was performed by using Pearson correlation test.
Results
The RA group was comprised of 40 patients; 20 were female and 20 male. To avoid the affect of ageing process (major contributor of CVD risk), middle age group (40-55 years) subjects were included. The mean age of RA patients was 48.7 years and disease duration was 32.9±5.8 months. RA patient population had a moderate disease activity with a mean DAS28 of 3.97±0.23. In the control group, 40 healthy volunteers of same age and sex matched subjects were recruited. There was no statistically significant difference between RA patients and controls with regard to age, gender, BMI and BP measurement (p<0.1) whereas ESR levels (32.8±3.95; p <0.001) were significantly high in patients group, as shown in [Table/Fig-1].
Demographic profile of Patient and Control groups (Mean±SD).
| Parameter | Control Group(n=40) | Patient group(n=40) | % Increase | % Decrease |
|---|
| Age (years) | 48.2 (5.3) | 48.7 (4.7) * | - | - |
| M:F ratio | 1:1 | 1:1 | - | - |
| Height (meter) | 1.59 (0.032) | 1.58 (0.028) | - | - |
| Weight (Kg) | 58.2 (1.8) | 61.9 (2.7) | - | - |
| BMI (Kg/m2) | 22.8 (1.3) | 24.3 (1.2)* | 6.58 % | - |
| Systolic blood pressure (mm Hg) | 107.25 (3.47) | 114.65 (4.30) | | |
| Diastolic blood pressure (mm Hg) | 74.6 (2.40) | 77.2 (2.47) | | |
| VAS (mm) | 0.0 | 35.02 (4.4) | - | - |
| ESR (mm/h) | 15.5 (2.24) | 32.8 (3.95)** | 111.6% | |
| DAS28 | 0.0 | 3.97 (0.24) | - | - |
where, * p < 0.1: Non-significant; ** p < 0.001 : Highly Significant, BMI, Body mass index; ESR, Erythrocyte sedimentation rate.
Existence of conventional risk factor for CVD, i.e. perturbed lipid profile pattern, in RA patient had supported the expected prediction, as shown in [Table/Fig-2]. Plasma total cholesterol (p <0.05), triglycerides (p <0.05), LDL cholesterol (p <0.001) and VLDL cholesterol (p <0.05) levels were found to be increased significantly, whereas serum HDL-cholesterol levels (p < 0.05) were decreased significantly in RA patients as compared to the values observed in controls. In addition, increased TC/ HDL-C ratio i.e. atherogenic index also revealed the increased risk of myocardial infarction in RA patients as this ratio was higher than five.
Plasma lipid profile of Patient and Control groups (Mean±SD).
| Parameter | Control Group(n=40) | Patient group(n=40) | % Increase | % Decrease |
|---|
| Total Cholesterol (mg/dl) | 155.96 ± 7.56 | 194.04 ± 10.73** | 24.41 | - |
| Triglycerides (mg/dl) | 106.02 ± 7.8 | 130.2 ± 9.2** | 22.80 | - |
| HDL cholesterol (mg/dl) | 46.6 ± 3.09 | 34.17 ± 3.27** | - | 26.67 |
| LDL cholesterol (mg/dl) | 92.68 ± 7.89 | 136.74 ± 7.34*** | 47.5 | - |
| VLDL cholesterol (mg/dl) | 21.80 ± 2.24 | 27.52 ± 2.48** | 26.2 | |
| Total cholesterol/HDL cholesterol ratio | 3.56 ± 0.73 | 5.39 ± 1.37*** | 51.4 | |
where, * * p < 0.1: Non-significant, ** p < 0.05: Significant, *** p < 0.001: Highly significant
HDL: High density lipoprotein; LDL: Low density lipoprotein.
VLDL: Very Low density lipoprotein
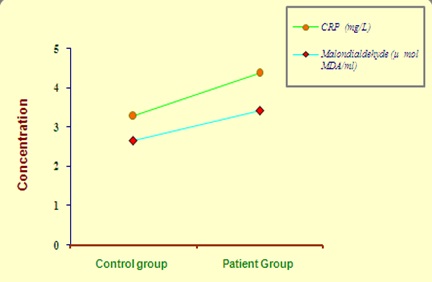
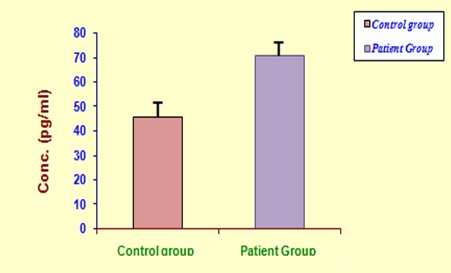
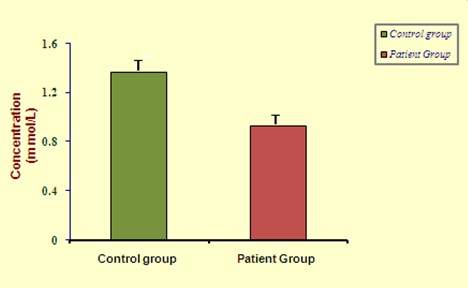
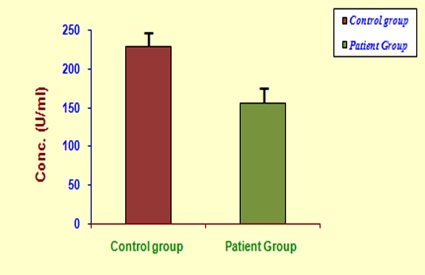
Plasma CRP (4.37 ± 0.14 vs 3.28 ± 0.10 mg/L; p < 0.05) and erythrocyte MDA (3.43 ± 0.16 vs 2.67 ± 0.17 μ mol MDA/ml; p < 0.001) were found to be increased significantly in RA subjects as compared to healthy controls [Table/Fig-3]. In addition, synovial IL-6 (71.05 ± 2.90 vs 45.85 ± 2.04 pg/ml; p < 0.001) levels were also found to be increased significantly in RA subjects [Table/Fig-4]. On the other hand, plasma TAA level (0.93 ± 0.09 vs 1.36 ± 0.14 m mol/L; p < 0.05; [Table/Fig-5] and PON activity (156.0 ± 2.18 vs 228.56 ± 9.56 U/ml; p < 0.001; [Table/Fig-6] were significantly low in RA group than that of controls.
Plasma CRP and erythrocyte MDA levels in study group subjects.
Synovial interleukin-6 levels in study group subjects.
Plasma total antioxidant activity levels in study group subjects.
Plasma paraoxonase activity levels in study group subjects.
In addition, we observed a negative correlation between PON and LDL cholesterol, MDA, VAS pain score, ESR, DAS28, disease duration of RA (DDRA) whereas HDL cholesterol and TAA levels were positively correlated with PON activity, as shown in [Table/Fig-7,8 and 9] respectively. Similarly, plasma PON activity was negatively correlated with plasma CRP (p < 0.05) and synovial fluid IL-6 levels (p < 0.05); as presented in [Table/Fig-9]. These results clarify the role of PON reduction in enhancing the CVD risk in RA patients most probably by its relation with dyslipidaemia, pain, oxidative stress and systemic inflammation including synovitis.
Correlation coefficient (r) between plasma paraoxonase activity (PON) and conventional markers (Lipid profile and BMI) of CVD in RA patients.
| Particulars | Total Cholesterol | TG | LDL-C | HDL-C |
|---|
| PON activity | -0.424 * | -0.381* | -0.690 ** | + 0.648 ** |
Where, * p < 0.05 : Significant, ** p < 0.001 : Highly significant
PON, Paraoxonase; LDL-C, Low density lipoprotein - cholesterol;
HDL-C, High density lipoprotein – cholesterol; TG, Triglyceride.
Correlation coefficient (r) between plasma paraoxonase activity (PON) and disease activity score -28(DAS28) along with other variables in RA patients.
| Particulars | VAS | ESR | DAS28 | DDRA |
|---|
| PON activity | - 0.584 ** | -0.610 ** | - 0.598 ** | -0.304 * |
Where, * p < 0.05 : Significant, ** p < 0.001 : Highly significant
PON, Paraoxonase; ESR, Erythrocyte sedimentation rate; VAS, Visual analog score; DAS28, Disease activity score 28; DDRA, Disease duration of Rheumatoid arthritis.
Correlation coefficient (r) between plasma paraoxonase activity (PON) and non-traditional markers (inflammation and oxidative stress) of CVD in RA patients.
| Particulars | SynovialIL-6 | Plasma CRP | Plasma TAA | MDA |
|---|
| PON activity | -0.426 * | - 0.458 * | + 0.608 ** | - 0.672 ** |
Where, * p < 0.05 : Significant, ** p < 0.001 : Highly significant
PON, Paraoxonase; IL-6, Interleukin-6; CRP, C- reactive protein; TAA, Total antioxidant activity; MDA, Malondialdehyde;.
Discussion
It is now well accepted that the presence of traditional risk factor merely, does not fully explain the mystery of CVD mortality in RA and reflects the need of identification of novel non-traditional risk factors. Growing evidences suggest that the involvement of inflammatory burden along with oxidative stress plays a crucial role in enhancing CVD risk in RA [1,7,23].
ROS produced by activated neutrophils during inflammatory reactions play an important role in the pathogenesis of many inflammatory diseases including CVD and RA [13,24]. Inflammatory reactions trigger oxidative stress that leads to lipid peroxidation of chondrocytes, mediates collagen degradation, promotes atherosclerotic plaque formation, prostacyclin synthesis, enhancement of cytosolic free calcium and peripheral vascular resistance leading to predisposition of CVD complications in RA [13,25]. In the present study, erythrocyte MDA levels were significantly high in RA subjects, which suggest the role of lipid peroxidation in RA progression and development of CVD risk. Altindag et al., in their study also reported that high MDA levels in RA patients were due to toxic effect of free radicals, generated as a result of oxidative stress [26].
PON, an antioxidant enzyme found in association with HDL contributing to its anti-atherogenic capability, not only regulates lipid peroxidation but it also acts to suppress inflammation [15]. In the present study, plasma PON activity (p<0.05) was found to be decreased significantly in RA subjects, which reflects toward the increasing susceptibility of RA patients to develop future CVD risk due to inability of system to control the enhanced systemic inflammation and production of reactive aldehydes via lipid peroxidation. It could be better explained on the basis of inactivation of enzyme itself due to interaction of oxidized lipids with the PON free sulphydryl group [27]. In addition, PON activity was inversely associated with LDL, TC and MDA levels, and directly associated with HDL which supports the association of dyslipidaemia and oxidative stress in enhancing the CVD risk in RA patients, and suggesting the maintenance of normal PON activity as one of the important event in CVD prevention in RA. Moreover, DAS28, duration of RA, ESR and VAS were inversely correlated with PON and appeared as independent predictors of PON status in RA patients [Table/Fig-8]. Our findings were in agreement with that of Altindag et al., who also reported that PON has anti-atherogenic capability and reduction in PON activity in RA increases the disease complexity [26]. Similarly, other reports in the medical literature have indicated an association of PON reduction with CVD risk factors in patients with different rheumatic diseases, such as systemic lupus erythematosus, osteoarthritis and psoriasis, etc [7,28,29]. However, plasma PON activity did not differ significantly in ankylosing spondylitis [30].
In addition, previous studies have emphasized the implication of a genetic T/C amino acid variant, found within the PON1 gene (RR192, QR192 or QQ192 genotype), in the prediction of CVD risk in RA [1,31]. According to Charles-Schoeman et al., PON1 activity values were found lower for the QQ genotype, intermediate for the QR genotype, and highest for the RR genotype in the RA patients [14]. Therefore, to validate the findings of the current study, study on PON1 gene with large sample size should be carried out to support the observations of the present study.
In general, alteration in TAA levels indicates the disturbance in the antioxidant defense system of the body due to oxidant overload. In the present study, plasma TAA levels were decreased significantly in RA patients as compared to controls and positively correlated with PON, which elucidate the combined effect of reduced antioxidant enzymes and non-enzymic antioxidants in RA pathology following the development of secondary complications due to augmented oxidative stress. Similarly, marked reduction in TAA levels in RA as well as in subjects with CVD risk have been well documented in earlier studies [26,32].
The physiology behind synovial inflammation and formation of unstable atherosclerotic plaque are same. In RA, T-lymphocytes infiltrate the synovial membrane and produce pro-inflammatory cytokines (such as IL-6, and TNF-α). These cytokines stimulate release of tissue-destroying matrix metalloproteinases, pro-inflammatory enzymes such as Cox-2, and prostaglandins. These enzymes then cause degeneration of cartilage extracellular matrix. In addition, IL-6 stimulates the synthesis of many acute-phase reactants, including CRP, which has emerged as an important predictor of myocardial infarction, stroke and vascular death [8,9,33–35]. In this context, our study demonstrates that patients with RA have marked increase in plasma CRP and synovial IL-6 levels as compared to controls [Table/Fig-3,4].
Moreover, inverse association of CRP, ESR and synovial IL-6 with PON supports the substantial involvement of inflammation in enhancing subclinical atherosclerotic complexity in RA pathology [Table/Fig-8,9]. Therefore, regulation of inflammation should be targeted not only to ameliorate the PON activity but also to overcome the burden of CVD risk in RA, as well documented by Popa et al., in RA patients, undergoing biologic therapy [36]. Consistent findings have been reported in previous study of arthritic patients suggesting CRP as an important marker of systemic inflammation along with other pro-inflammatory cytokine (IL-6), and have a role in the disease pathology [23]. Recently, Navarro-Millan et al., also reported that elevated CRP, erythrocyte sedimentation rate and dyslipidaemia, as observed in present study, predict CVD risk in patients with RA [2]. Conversely, Shadick et al., documented that measurement of CRP alone, does not significantly predict subsequent risk of RA in women [37].
Limitations
The inability to follow up the subjects with rheumatoid arthritis, due to paucity of time and limited resources.
Conclusion
On the basis of present study findings, it is apparent that patients with RA have low plasma PON activity due to inflammation mediated oxidant overload and, thus, may predisposed to an advanced risk for the development of CVD. In addition to IL-6, plasma PON activity, CRP and TAA levels could be considered as non-traditional factors to predict CVD risk. Therefore, supplementation of diet rich in antioxidants along with Disease Modifying Anti-Rheumatic Drugs (DMARD) may be an effective approach in the prevention of CVD risk as well as RA progression. Furthermore, adoption of preventive measures and development of future drugs are obligatory to reduce not only non-traditional factors such as inflammation and oxidative stress but also conventional CVD risk factors, which collectively constitute the chief contributors to the global burden of both of these diseases. Further investigations and multicenter studies with large sample size should be carried out to support current view point and to provide substantial understanding of these inflammatory diseases, on molecular level, so that the incidence of aforesaid risk can be abridged before it is too late.
where, * p < 0.1: Non-significant; ** p < 0.001 : Highly Significant, BMI, Body mass index; ESR, Erythrocyte sedimentation rate.
where, * * p < 0.1: Non-significant, ** p < 0.05: Significant, *** p < 0.001: Highly significant
HDL: High density lipoprotein; LDL: Low density lipoprotein.
VLDL: Very Low density lipoprotein
Where, * p < 0.05 : Significant, ** p < 0.001 : Highly significant
PON, Paraoxonase; LDL-C, Low density lipoprotein - cholesterol;
HDL-C, High density lipoprotein – cholesterol; TG, Triglyceride.
Where, * p < 0.05 : Significant, ** p < 0.001 : Highly significant
PON, Paraoxonase; ESR, Erythrocyte sedimentation rate; VAS, Visual analog score; DAS28, Disease activity score 28; DDRA, Disease duration of Rheumatoid arthritis.
Where, * p < 0.05 : Significant, ** p < 0.001 : Highly significant
PON, Paraoxonase; IL-6, Interleukin-6; CRP, C- reactive protein; TAA, Total antioxidant activity; MDA, Malondialdehyde;.