Disseminated Histoplasmosis with Haemophagocytic Lymphohistiocytosis in an Immunocompetent Host
Amey Dilip Sonavane1, Pratibha Balasaheb Sonawane2, Sachet Vijay Chandak3, Pravin M Rathi4
1 Gastroenterology Fellow, Department of Gastroenterology, Bombay Hospital and Institute of Medical Sciences, Mumbai, India.
2 Gastroenterology Fellow, Department of Gastroenterology, Bombay Hospital and Institute of Medical Sciences, Mumbai, India.
3 Gastroenterology Fellow, Department of Gastroenterology, Bombay Hospital and Institute of Medical Sciences, Mumbai, India.
4 Consultant Gastroenterologist, Department of Gastroenterology, Bombay Hospital and Institute of Medical Sciences, Mumbai, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Amey Dilip Sonavane, A/101, Shakuntala-Krupa C.H.S.; Near Mental Hospital Gate, Wagle Estate Post Office, Thane (West), Maharashtra-400604, India. E-mail : amey_max@yahoo.com
Haemophagocytic lymphohistiocytosis (HLH) is a devastating syndrome due to uninhibited immune activation. Disseminated histoplasmosis is a rare cause of HLH. There have been few case reports and series demonstrating a relation between the two disease entities in immunosuppressed hosts. HLH secondary to disseminated histoplasmosis is even rarer in an immunocompetant host. We report a rare case of HLH triggered by disseminated histoplasmosis in an immunocompetant patient.
Clinical infectious disease, Haemophagocytosis, Invasive fungal infections
Case Report
A 43-year-old lady presented to the Department of Gastroenterology with complaints of intermittent high grade fever with chills since seven months. She had a negative workup for malaria, dengue and typhoid fever and her blood culture did not grow any pathogenic organism. She also complained of loss of weight and appetite alongwith generalized fatigability. Six months back, she was diagnosed with pulmonary tuberculosis (TB) {sputum Acid Fast Bacillus (AFB) positive, chest radiograph suggestive of bilateral upper zone infiltrative shadows} and received treatment under Directly Observed Treatment, Short-course (DOTS). Her sputum AFB was negative at three months, five months and end of treatment. However, she continued to lose weight (five kilograms over six months) and complained of generalized weakness and persistent loss of appetite. Since, two months prior to presentation, she complained of gradually progressive abdominal distension and dull, dragging, continuous, non-radiating, bilateral hypochondriac pain. She had early satiety, joint pains and continued to have fever with chills. She developed swelling over both legs and a nonproductive cough. She had no major medical and surgical co-morbidities other than recently treated TB. She was a homemaker and had two healthy children. On examination, she had mild fever (99o Fahrenheit), pallor, bilateral pitting pedal oedema and gross hepatosplenomegaly. Both the liver and spleen were firm, nontender and had a smooth surface and rounded margins. The liver span was 20 cm and the spleen had enlarged upto the umbilicus with a palpable splenic notch.
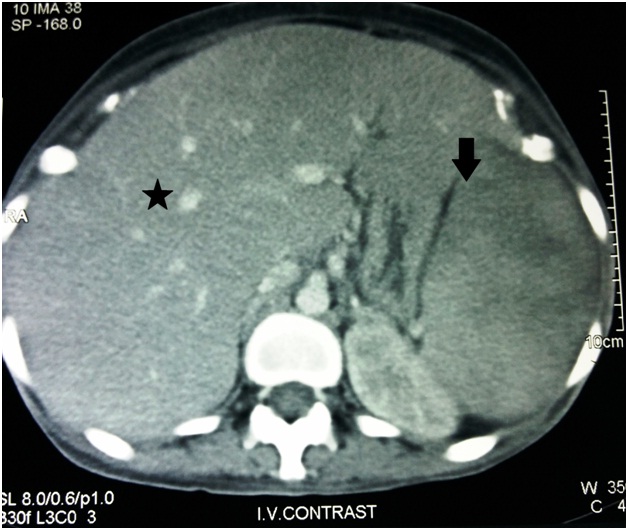
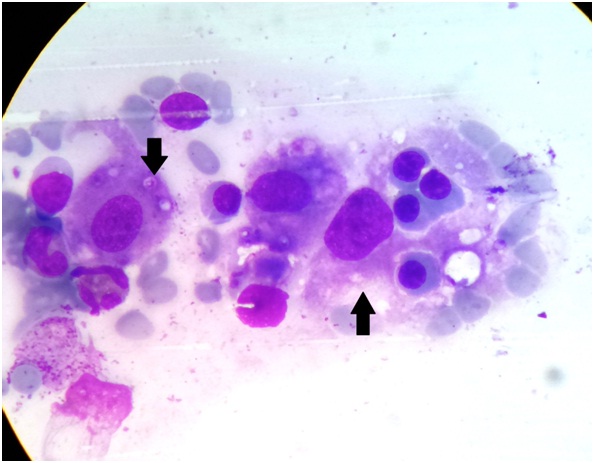
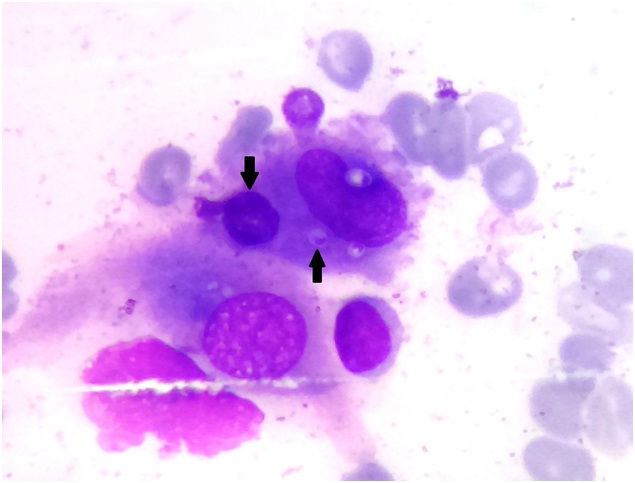
Laboratory evaluation revealed pancytopenia, low serum albumin with albumin-globulin ratio reversal, raised serum lactate dehydrogenase and serum alkaline phosphatase levels [Table/Fig-1,2]. She underwent an ultrasound of the abdomen that revealed moderate hepatomegaly with altered echotexture without any biliary obstructive changes, gross splenomegaly, no ascites and few small peripancreatic lymph nodes. A Computerized Tomogram (CT) of the chest was performed for new onset dry cough which revealed lesions in bilateral upper lung lobes representing resolving tuberculous infection and hepatosplenomegaly [Table/Fig-3a,b,4]. Sputum for AFB smear (2 samples) was negative and TB Gene Xpert did not detect Mycobacterium Tuberculosis. Antinuclear antibody test by immunofluorescence technique was negative. Tests for Human Immunodeficiency virus (HIV), antibody to Hepatitis C virus (Anti-HCV) and Hepatitis B virus surface antigen (HBsAg) were non reactive. In view of persistent pancytopenia, gross splenomegaly and continual fever despite adequate TB treatment, a bone marrow examination was performed. It revealed a mildly hypercellular marrow showing panmyelosis, histiocytes showing presence of Histoplasma capsulatum and occasional erythrophagocytosis [Table/Fig-5,6]. Methanamine Silver staining revealed few small round to oval, occasionally budding, yeast forms of Histoplasma capsulatum. She received Amphotericin B deoxycholate for 14 days followed by Itraconazole therapy. At the end of 14 days, the liver and spleen had regressed in size and she was asymptomatic. At one month follow-up, she was completely symptom-free and was tolerating Itraconazole well.
Serial complete blood counts.
| Laboratory Parameter | Month Prior to Admission | At Admission | One week after completion of treatment | Two weeks after completion of treatment | At Discharge |
|---|
| Hb (g/dl) | 7.6 | 7.8 | 7.0 | 9.2 | 10.4 |
| Packed Cell Volume (%) | 23.2 | 24.3 | 22.4 | 29 | 31 |
| Mean Corpuscular Volume (cu-microns) | 71.38 | 73.86 | 77.6 | 81.3 | 83.4 |
| Total Leucocyte Count (cu-microns) | 3600 | 2800 | 2600 | 2700 | 5400 |
| Red Cell Diameter Width (%) | 19.5 | 19.2 | 22.4 | 22.2 | 20.6 |
| Platelet Count (/mm3) | 116000 | 54000 | 91000 | 121000 | 163000 |
Other laboratory parameters.
| Laboratory parameter | Value | Reference Range |
|---|
| Bilirubin (Total) | 0.5 mg% | (0.0 mg% -1.0 mg%) |
| Bilirubin (Direct) | 0.2 mg% | (0.0 mg% -0.3 mg%) |
| Total Proteins | 5.6 g% | (6.4 g% - 8.2 g%) |
| Serum Albumin | 1.6 g% | (3.4 g% - 5.0 g%) |
| Serum Globulin | 4.0 g% | (2.8 g% - 3.6 g%) |
| Serum Cholesterol | 67 mg% | (125 mg% - 200 mg%) |
| ALT | 23 mU/ml | (15 mU/ml – 63 mU/ml) |
| AST | 28 mU/ml | (15 mU/ml – 37 mU/ml) |
| Alkaline Phosphatase | 277 mU/ml | (50 mU/ml – 136 mU/ml) |
| GGTP | 69 mU/ml | (5mU/ml – 85 mU/ml) |
| Serum Ferritin | 891.7 ng/ml | (13 ng/ml – 150 ng/ml) |
| Serum Creatinine | 0.9 mg% | (0.6 mg% - 1.3 mg%) |
| Serum Triglycerides | 89 mg% | (30 mg% - 200 mg%) |
| Serum Fibrinogen | 53 mg/dl | (180 mg/dl – 350 mg/dl) |
| Serum Lactate Dehydrogenase | 296 mU/ml | (81 mU/ml – 234 mU/ml) |
| Thyroid Stimulating Hormone | 3.68 uIU/ml | (0.2 uIU/ml – 6 uIU/ml) |
| Fasting Blood Sugar | 109 mg/dl | (70 mg/dl – 120 mg/dl) |
| Erythrocyte Sedimentation Rate | 19 mm/hr | (0 mm/hr - 20 mm/hr) |
A CT image of chest (coronal and sagittal) suggestive of fibrotic strands in bilateral upper lobes demonstrating healed pulmonary tuberculosis
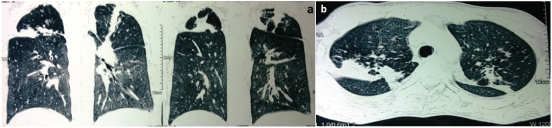
A CT image of Abdomen suggesting Hepatomegaly (Star) and Splenomegaly (Down Arrow)
A Haematoxylin-Eosin photomicrograph of bone marrow aspirate revealing an erythrophagocytic cell devouring a red cell (Up Arrow) and multiple intracytoplasmic Histoplasma capsulatum (Down Arrow)
A Haematoxylin-Eosin photomicrograph of bone marrow aspirate revealing an erythrophagocytic cell devouring multiple red blood cells (Down Arrow) and multiple intracytoplasmic Histoplasma capsulatum (Up Arrow)
Discussion
Haemophagocytic lymphohistiocytosis (HLH) is a life-threatening disease characterized by fever, jaundice, splenomegaly and the pathologic finding of haemophagocytosis (destruction of erythrocytes, leukocytes, platelets and their precursors by macrophages) in bone marrow and other tissues. A “cytokine storm” caused by uncontrolled proliferation of activated lymphocytes and macrophages results in severe inflammation. Primary HLH, seen mostly in children, is triggered by primary genetic disorders and is a heterogeneous autosomal recessive disorder. The underlying defect in cytotoxic functioning of natural killer (NK) and T lymphocytes results in secretion of large quantities of cytokines. In adults, hematologic malignancies, autoimmune diseases, bacterial, fungal and viral infections can trigger “secondary HLH” [1,2]. Common laboratory anomalies seen in HLH are anaemia, thrombocytopenia, neutropenia, hyperferritinemia, hypertriglyceridemia, hypofibrinogenemia and hyperbilirubinemia.
Temporary acquired immune-suppressant states also cause NK cell defects leading to secondary HLH. Organisms causing intracellular infection can hence trigger HLH and these include viruses (Epstein-Barr virus (EBV), Cytomegalovirus, Human Immunodeficiency Virus, Parvovirus B19, Dengue, Varicella-Zoster, Herpes Simplex Virus), parasites (Malaria sp., Babesia sp. Leishmania sp., Toxoplasma gondii), mycobacteria, fungi (Cryptococcus neoformans, Histoplasma Capsulatum, Aspergillus, Penicillium marneffei) and bacteria (Salmonella sp., Rickettsia sp., Leptospira sp., Brucella sp., Borrelia sp., Bartonella sp., Listeria sp., Coxiella). A “double hit” of ineffective clearance of infection and a hyper immune response causes extensive tissue damage. One study looked at HLH triggered by tropical infections from the Indian sub-continent, among adults. The most common tropical infectious diseases triggering more than 50% of the cases of HLH were visceral leishmaniasis; rickettsial infection; malaria; histoplasmosis; enteric fever and tuberculosis. Viral agents triggered another 30% (most often EBV and Parvovirus B19) [3].
Four types of infection caused by histoplasmosis have been described in literature: primary pulmonary histoplasmosis, primary cutaneous histoplasmosis, progressive disseminated histoplasmosis and African histoplasmosis [4]. Progressive disseminated histoplasmosis, especially seen in an immunocompromised host is an uncommon disease in India. Inhalation of soil mixed with bird and bat droppings is the most common source of infection. Diagnosis is established by demonstrating the organism in an extrapulmonary location. Thirty eight cases of Histoplasmosis associated HLH have been reported worldwide, including the largest case series (11 cases) published recently [5]. Eight cases of disseminated histoplasmosis with HLH have been reported from India largely in immunosuppressed hosts [6–11]. Four of them had a fulminant disease and died, one had underlying HIV infection and another three was treated successfully with antifungal therapy. Only two patients were immunocompetant. This is thus a rare case of HLH complicating disseminated histoplasmosis in an immunocompetant host reported from India. All cases of disseminated histoplasmosis with HLH reported in literature over the last 10 years with their clinical outcome have been summarized in a tabulated form [Table/Fig-7] [12–25].
The table enlists prominent cases of disseminated histoplasmosis with HLH published in literature over the last 10 years
| Author | Year of Publication | Co-morbidities/Immune Status | Survival |
|---|
| Gil-Brusola [12] | 2007 | HIV | Died |
| Wang [13] | 2007 | Chronic Hepatitis C, Cryoglobulinemia, Chronic Kidney Disease and Fungal endocarditis | Died |
| Guiot [14] | 2007 | HIV | Survived |
| Sanchez [15] | 2007 | HIV | Survived |
| Phillips [16] | 2008 | Sarcoidosis on chronic steroids | Survived |
| De Lavaissiere [17] | 2009 | HIV | Survived |
| Van Koeveringe [18] | 2010 | Chronic Lymphocytic Leukemia | Survived |
| Lo [19] | 2010 | Renal transplant | Survived |
| Vaid [7] | 2011 | HIV | Died |
| Chandra [8] | 2012 | HIV | Survived |
| Nieto-Rivers [20] | 2012 | Renal Transplant | Survived |
| Nieto-Rivers [20] | 2012 | Renal Transplant | Died |
| Telfer M [21] | 2012 | HIV | Died |
| Alina M Huang [22] | 2014 | HIV | Survived |
| Ashish Rajput [23] | 2015 | Scleroderma, Monoclonal Gammopathy of Undetermined Significance | Survived |
| M Kashif [24] | 2015 | Sickle cell anaemia | Died |
| A Subedee [25] | 2015 | HIV | Died |
| T Mukherjee [10] | 2015 | Chronic Obstructive Pulmonary Disease, Erythema Nodosum | Died |
| De [11] | 2015 | Healthy | Survived (Patient 1) |
| De [11] | 2015 | Healthy | Survived (Patient 2) |
| A Sonavane (Present Report) | 2015 | Healthy | Survived |
Clinical Practice Guidelines [26] for the management of patients with histoplasmosis (2007) by the Infectious Diseases Society of America recommends liposomal Amphotericin B (3.0 mg/kg daily) for 1–2 weeks, followed by oral itraconazole (200 mg 3 times daily for 3 days and then 200 mg twice daily for a total of at least 12 months) for moderately severe to severe disease. The deoxycholate formulation of Amphotericin B (0.7–1.0 mg/kg daily) is an alternative to a lipid formulation in patients who are at a low risk for nephrotoxicity. For mild-to-moderate disease, itraconazole (200 mg 3 times daily for 3 days and then twice daily for at least 12 months) is recommended. Lifelong therapy with itraconazole (200 mg daily) may be required in immunosuppressed patients if immunosuppression cannot be reversed and in patients who relapse despite receiving appropriate antifungal therapy.
Conclusion
Our patient satisfied both the HLH-2004 criteria for diagnosis of HLH and Infectious Diseases Society of America Guidelines for diagnosis of histoplasmosis. Disseminated Histoplasmosis as a cause of haemophagocytic syndrome is a very rare syndrome that has been described in only a handful of cases in the literature and most of them had an underlying immunosuppressed host. However, HLH with histoplasmosis is extremely rare in an immunocompetant individual. The patient was treated adequately for pulmonary tuberculosis as the initial clinical picture was consistent and she had sputum AFB positivity. However, as fever and hepatosplenomegaly did not revert with adequate TB treatment, search for a possible second infection or malignancy prompted a bone marrow examination that uncovered histoplasmosis. She was adequately treated with the recommended antifungals and had a favourable outcome. Well-timed diagnosis and timely treatment is the cornerstone of management as the disease, if left untreated can prove fatal.
[1]. Rouphael NG, Talati NJ, Vaughan C, Infections associated with haemophagocytic syndromeLancet Infect Dis 2007 7:814-22. [Google Scholar]
[2]. Filipovich AH, The expanding spectrum of haemophagocytic lymphohistiocytosisCurr Opin Allergy ClinImmunol 2011 11:512-16. [Google Scholar]
[3]. Rajagopala S, Singh N, Diagnosing and treating haemophagocytic Lymphohistiocytosis in the tropics: Systematic review from the Indian subcontinentActa Medica Academica 2012 41:161-74. [Google Scholar]
[4]. James William D, Berger Timothy G, Andrews’ Diseases of the Skin: clinical Dermatology 2006 Saunders ElsevierISBN 0-7216-2921-20 [Google Scholar]
[5]. Townsend JL, Shanbhag S, Hancock J, Bowman K, Nijhawan AE, Histoplasmosis-Induced Hemophagocytic Syndrome: A Case Series and Review of theLiteratureOpen Forum Infect Dis 2015 2(2):ofv055doi: 10.1093/ofid/ofv055 [Google Scholar]
[6]. Kumar N, Jain S, Singh ZN, Disseminated histoplasmosis with reactive haemophagocytosis: aspiration cytology findings in two casesDiagn Cytopathol 2000 23:422-24. [Google Scholar]
[7]. Vaid N, Patel P, A case of haemophagocytic syndrome in HIV associated disseminated histoplasmosisAcute Med 2011 10:142-44. [Google Scholar]
[8]. Chandra H, Chandra S, Sharma A, Histoplasmosis on bone marrow Aspirate: cytological examination associated with haemophagocytosis and pancytopenia in an AIDS patientKorean J Hematol 2012 47:77-79. [Google Scholar]
[9]. Saluja S, Sunita Bhasin S, Gupta DK, Gupta B, Kataria SP, Disseminated histoplasmosis with reactive haemophagocytosis presenting as PUO in an immunocompetent hostJ Assoc Physicians India 2005 53:906-08. [Google Scholar]
[10]. Mukherjee T, Basu A, Disseminated histoplasmosis presenting as a case of erythema nodosum and haemophagocytic LymphohistiocytosisMJAFI(in press) [Google Scholar]
[11]. De D, Nath UK, Disseminated Histoplasmosis in Immunocompetent Individuals- not a so Rare Entity, in IndiaMediterranean Journal of Hematology and Infectious Diseases 2015 7(1):e2015028 [Google Scholar]
[12]. Gil-Brusola A, Peman J, Santos M, Disseminated histoplasmosis with haemophagocytic syndrome in a patient with AIDS: description of one case and review of the Spanish literatureRev Iberoam Micol 2007 24:312-16. [Google Scholar]
[13]. Wang Z, Duarte AG, Schnadig VJ, Fatal reactive haemophagocytosis related to disseminated histoplasmosis with endocarditis: an unusual case diagnosed at autopsySouth Med J 2007 100:208-11. [Google Scholar]
[14]. Guiot HM, Bertran-Pasarell J, Tormos LM, Ileal perforation and reactive haemophagocytic syndrome in a patient with disseminated histoplasmosis: the role of the real-time polymerase chain reaction in the diagnosis and successful treatment with Amphotericin B lipid complexDiagn Microbiol Infect Dis 2007 57:429-33. [Google Scholar]
[15]. Sanchez A, Celaya AK, Victorio A, Histoplasmosis-associated haemophagocytic syndrome: a case reportAIDS Read 2007 17:496-99. [Google Scholar]
[16]. Phillips J, Staszewski H, Garrison M, Successful treatment of secondary haemophagocytic lymphohistiocytosis in a patient with disseminated histoplasmosisHaematology 2008 13:282-85. [Google Scholar]
[17]. De Lavaissiere M, Manceron V, Bouree P, Reconstitution inflammatory syndrome related to histoplasmosis, with a haemophagocytic syndrome in HIV infectionJ Infect 2009 58:245-47. [Google Scholar]
[18]. Van Koeveringe MP, Brouwer RE, Histoplasma capsulatum reactivation with haemophagocytic syndrome in a patient with chronic lymphocytic leukaemiaNeth J Med 2010 68:418-21. [Google Scholar]
[19]. Lo MM, Mo JQ, Dixon BP, Czech KA, Disseminated histoplasmosis associated with haemophagocytic lymphohistiocytosis in kidney transplant recipientsAm J Transplant 2010 10:687-91. [Google Scholar]
[20]. Nieto-Rivers JF, Aristizabal-Alzate A, Ocampo C, Serrano-Gayubo AK, Serna-Higuita LM, Valencia GZ, Haemophagocytic syndrome and disseminated histoplasmosis in two kidney transplant patientsNefrologia 2012 32:683-84. [Google Scholar]
[21]. Telfer M, Gulati S, Haemophagocytic lymphohistiocytosis in a patient with disseminated histoplasmosis: a case reportJournal of Hospital Medicine 2012 7(2) [Google Scholar]
[22]. Huang AM, Haemophagocytic lymphohistiocytosis and disseminated histoplasmosisBlood 2014 123(16):2449 [Google Scholar]
[23]. Rajput A, Bence-Bruckler I, Huebsch L, Jessamine P, Toye B, Padmore R, Disseminated histoplasmosis associated with acquired haemophagocytic LymphohistiocytosisClinical Case Reports 2015 3(3):195-96. [Google Scholar]
[24]. Kashif M, Tariq H, Ijaz M, Gomez-Marquez J, Disseminated histoplasmosis and secondary haemophagocytic syndrome in a non-hiv patientCase Reports in Critical Care 2015 2015:295735 [Google Scholar]
[25]. Subedee A, Sickels NV, Haemophagocytic Syndrome in the Setting of AIDS and disseminated histoplasmosis: case report and a review of literatureJ Int Assoc Provid AIDS Care 2015 14:391-97. [Google Scholar]
[26]. Joseph Wheat L, Freifeld AG, Kleiman MB, Baddley JW, McKinsey DS, Loyd JE, Clinical Practice Guidelines for the Management of Patients with Histoplasmosis: 2007 Update by the Infectious Diseases Society of AmericaClin Infect Dis 2007 45(7):807-25. [Google Scholar]