Type 2 diabetes mellitus is considered as a heterogeneous syndrome of dysregulated glucose homeostasis. The world prevalence of diabetes in adults has been predicted to increase from 285 million in 2010 to 439 million in 2030 [1]. It is also increasingly recognized that adequate management of diabetes by appropriate drug therapy is essential in preventing complications.
The peroxisome proliferator–activated receptor γ gene (PPARG) encodes the PPARγ receptor, a transcription factor that belongs to the family of nuclear receptors and it regulates the carbohydrate and lipid metabolism. Among the two isoforms, PPAR-γ2 is specific for adipose tissue, where it plays a pivotal role in adipogenesis and is an important mediator of insulin sensitivity [2].
Pro12Ala polymorphism is the result of a CCA-to-GCA missense mutation in the codon12 of exon B of the PPARG gene. There is substantial evidence from various genetic studies that the Pro12Ala polymorphism improves insulin sensitivity in humans [3,4]. It is possible that alterations in transcriptional activity of the polymorphic gene in adipocytes primarily enhance insulin’s action [4,5]. Pioglitazone is a thiazolidinedione, the pharmacological ligand for PPARγ receptor. The thiazolidinediones act by decreasing the insulin resistance in muscle, liver and adipose tissue.
The effect of Pro12Ala polymorphism (rs1801282) on the therapeutic response to pioglitazone has not yet been studied in the South Indian population. Hence our study was designed to investigate the association of PPARG gene Pro12Ala polymorphism and the therapeutic response to pioglitazone therapy in type 2 diabetes mellitus patients in a South Indian population. The secondary objectives were to determine the influence of patient characteristics like body weight, BMI, waist-hip ratio on glycaemic response to pioglitazone and also to identify the association between Pro12Ala polymorphism and these patient characteristics in the population studied.
Materials and Methods
The present study was a hospital based prospective pilot study done in 30 patients. The study protocol was approved by the Institutional Human Ethics Committee before the start of the study. Patients who had already been diagnosed with type 2 diabetes mellitus and on regular treatment were included for the study. As per the current American Diabetes Association (ADA) guidelines, fasting plasma glucose ≥ 126 mg/dl or 2 hour postprandial plasma glucose ≥ 200 mg/dl or HbA1C ≥ 6.5% is the threshold for the diagnosis of diabetes [6].
Diabetic patients aged 30–85 years, of both sexes and already on treatment with sulfonylurea or metformin, but without adequate glycaemic control, as evidenced by HbA1C values of 7.5 to 9.5 % were selected for the study. Only those patients whose diabetic medications were not changed in the previous six months and those who had no previous history of PPAR agonist use were included.
Patients with type1 diabetes and known history of ischemic heart disease or congestive cardiac failure were excluded. Similarly known cases of liver disease, bladder cancer or previous history of fractures were excluded from the study. Patients already on drugs which increase fluid retention like steroids, NSAIDs as well as pregnant and lactating women were excluded.
Written informed consent was obtained from each of the patients who met the above criteria. Basic demographic data like name, age, sex and anthropometric measurements of height, weight, body mass index, waist circumference, hip circumference and waist hip ratio were recorded. Baseline fasting blood glucose levels (by enzymatic reference method) and HbA1C levels (by immunoturbidimetry) were also determined during the first visit. Pioglitazone 30 mg once daily was added to the existing regimen without changing any other medication. The patients were counseled for self reporting of any adverse events. They were also instructed to maintain the same level of energy intake and physical activity as before. The next visit was scheduled after 12 weeks, during which the body weight was recorded in addition to repeating the fasting blood glucose and HbA1C. There were no significant adverse drug reactions reported by the participants during the study period.
The expected average reduction in HbA1C in type 2 diabetic patients with pioglitazone therapy is in the range of 0.5 to 1.4% [7]. Glycaemic response to pioglitazone was determined by the fall in HbA1C after 12 weeks of add-on pioglitazone therapy. If the absolute decrease in HbA1C was less than 0.5%, they were categorized as non-responders. During the same visit, blood was also drawn for genetic analysis.
Genotyping of the patients was performed to identify the Pro12Ala polymorphism in the PPAR gamma gene. The genomic DNA was first extracted from the blood samples of the study participants. The extracted DNA was rinsed with 70% alcohol and re-suspended in 40 μL of TE buffer (pH 8.0). Pro12Ala polymorphism was determined by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). The DNA was amplified by PCR using a sense primer (5’-GCCAATTCAAGCCCAGTC-3’) and an antisense primer (5’-GATATGTTTGCAGACAGTGTATCAGTGAAGGAATCGCTTTCCG-3’) that flanked the region containing the 12-amino acid site of PPARγ2. The PCR product was 270 bp in length. The PCR conditions were an initial denaturation step at 94°C for 8 min, followed by 35 cycles of denaturation at 94°C for 50 sec, annealing at 59°C for 50 sec, and extension at 72°C for 1 min, with a final extension of 5 min at 72°C.
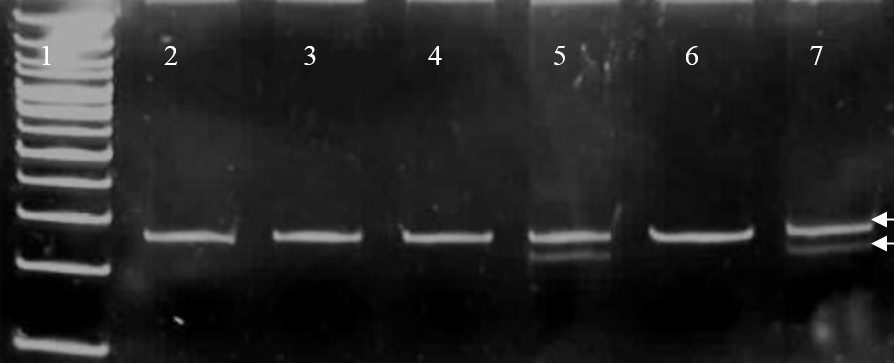
After PCR amplification, the PCR product was subjected to restriction digestion by BstUI/Bsh 1236 I (Fermentas 10units/ μL) enzyme at 37°C overnight, electrophoresed on a 12% polyacrylamide gel and stained with ethidium bromide. The digested RFLP products after gel electrophoreses was used to identify the study subjects with the Pro12Ala polymorphism. A single 270 bp fragment indicated the presence of CC (Pro12Pro) genotype while three fragments of 270, 227 and 43 bp product size confirmed the presence of GC (Pro12Ala) genotype [Table/Fig-1]. GG (Ala12Ala) genotype would be represented by two fragments of 227 and 43 bp [8]. Inorder to ensure validity, the samples were run in polyacrylamide gel using an internal positive and negative control and replicating 10% of the samples randomly. In addition, the gel was read by two independent blinded observers and the concordance rate was found to be 100%.
PCR-Restriction fragment length polymorphism detection of Pro12Ala missense mutation.
Lane1 shows the DNA ladder. Lanes 2,3,4,6 show the uncut wild type Pro12Pro genotype with single DNA fragment of product of size 270 bp (base pairs). Lanes 5,7 show the two DNA fragments of size 270 bp (upper arrow) and 227 bp (lower arrow) resulting from the restriction site introduced by the downstream primer due to Proline →Alanine substitution (Pro12Ala polymorphism). The 43bp DNA fragment is not seen.
Statistical Analysis
The data was analysed using the SPSS version 19. All data were expressed as mean± standard deviation. Paired t-test was performed to assess the effect of pioglitazone treatment and an independent sample t-test to analyse if there was any significant difference in the body weight, BMI, waist circumference, waist-hip ratio, baseline fasting blood glucose or the baseline HbA1C between wild type (homozygous) and those with Pro12Ala polymorphism (heterozygous). Logistic regression analysis was used to evaluate the associations between age, baseline body weight, BMI, waist circumference, waist-hip ratio and Pro12Ala variants with the response to pioglitazone. The p-value< 0.05 was considered significant.
Results
The mean age of the study participants was 53.2 ± 10.95 years, of which 46% were males and 54% females. At baseline, the mean body weight of the study population was 66.83 ± 10.492 kg and the mean BMI 26.247 ± 3.71. The mean waist circumference and mean waist-hip ratio were 90.50 ± 9.343 cm and 0.9033 ± 0.0649 respectively. Using ‘paired t-test’, a significant decrease in mean fasting blood glucose and HbA1C levels was seen after 12 weeks of add-on treatment with pioglitazone whereas mean body weight and BMI did not change significantly [Table/Fig-2]. The PPARγ (rs1801282) genotype frequency distributions were 80% for Pro/Pro, 20% for Pro/Ala and 0% for Ala/Ala in the study population. An independent sample t-test [Table/Fig-3] showed that there was no statistically significant difference found in the body weight, BMI, waist circumference, waist-hip ratio, fasting blood glucose or HbA1C between wild type (homozygous) and those with Pro12Ala polymorphism (heterozygous) at baseline. Interestingly, after pioglitazone treatment, the fasting blood glucose between wild type (125.92 ± 21.549) and heterozygous (106.33 ± 5.645) was statistically significant with p-value of 0.03. A similar statistically significant difference in HbA1C between the wild type (7.158 ± 1.007) and heterozygous (6.300 ± .4690) genotypes was found. However, the difference in other parameters after treatment was not statistically significant.
Among the study participants, 30% were non-responders and 70% responders to pioglitazone add-on therapy. At baseline, 22 patients in the study were receiving metformin while the rest were on treatment with sulfonylureas, among which, five patients were on glimepride and three on glibenclamide treatment. Also, it was noted that amongst those with the Pro12Ala polymorphism, five were on pre-existing metformin therapy while only one patient was on glibenclamide therapy.
Logistic regression analysis performed showed that there was no statistically significant association between the age, BMI, waist circumference or waist-hip ratio with the glycaemic response to pioglitazone therapy in the study population. But a statistically significant association was detected both for baseline bodyweight of the patients and PPARγ genotype Pro12Ala with the therapeutic response to pioglitazone [Table/Fig-4]. An analysis based on different pre-existing treatment regimes of the patients was not done for two reasons. First, we had included only those patients whose treatments were not changed in the previous six months implying that any change in their glycaemic response after the addition of pioglitazone was due to the add-on treatment. In addition, the smaller sample in the sulfonylurea group precluded direct comparison with the metformin group.
Paired t-test showing significant difference in fasting and HbA1C before and after 12 weeks pioglitazone treatment.
| Parameter | Mean ± Standard deviation | Mean ± Standard deviation (paired difference) | 95% CI | t value | p-value |
|---|
| Weight (baseline) | 66.83 ± 10.492 | -0.300 ± 1.055 | -0.694 - 0.094 | -1.557 | 0.13 |
| Weight (after 12 weeks) | 67.13 ± 10.536 |
| BMI (baseline) | 26.24 ± 3.774 | -0.017 ± 0.7968 | -0.3145 - 0.2805 | -0.117 | 0.9 |
| BMI (after 12 weeks) | 26.30 ± 3.710 |
| Fasting blood glucose (baseline) | 144.17 ± 13.608 | 22.167 ± 10.107 | 18.393 - 25.941 | 12.013 | <0.001 |
| Fasting blood glucose (after 12 weeks) | 122.00 ± 20.910 |
| HbA1C (baseline) | 7.770 ± 0.6199 | 0.7833 ± 0.4348 | 0.6210 - 0.9457 | 9.868 | <0.001 |
| HbA1C (after 12 weeks) | 6.987 ± 0.9825 |
Comparison of baseline patient characteristics in type 2 diabetes with Pro12Pro and Pro12Ala genotypes.
| Characteristics | Pro12Pro | Pro12Ala | p-value |
|---|
| Body weight | 68.04 ± 9.976 | 62.00 ± 12.066 | 0.2 |
| BMI | 26.695 ± 3.7858 | 24.458 ± 3.0170 | 0.1 |
| Waist circumference | 91.33 ± 8.746 | 87.17 ± 11.737 | 0.3 |
| Waist-hip ratio | 0.9046 ± 0.0678 | 0.8983 ± 0.0570 | 0.8 |
| Fasting blood glucose | 146.08 ± 14.222 | 136.50 ± 7.450 | 0.1 |
| HbA1C | 7.854 ± 0.6527 | 7.433 ± 0.3141 | 0.1 |
Multiple logistic regression analysis for the influence of clinical and genetic factors on the response to pioglitazone treatment.
| Variable | Odds ratio | p-value |
|---|
| Age | 0.015 | 0.90 |
| Body Weight | 4.933 | 0.02 |
| BMI | 3.199 | 0.07 |
| Waist circumference | 0.985 | 0.30 |
| Waist hip ratio | 2.717 | 0.09 |
| Pro12Ala (rs1801282) | 7.763 | 0.005 |
Discussion
In this study, the Pro12Ala polymorphism has been detected in 20% of the study population. This is similar to the frequency of the polymorphism reported in other studies [9–12] done in the Indian population. The current study has illustrated that there is no significant difference in the body weight between the wild type and heterozygous population [Table/Fig-3]. Likewise, there is no statistically significant association between the polymorphism and parameters of adiposity like BMI, waist circumference and waist hip ratio. Various studies done in different ethnic populations have established that the effect of Pro12Ala polymorphism on body mass index is complex. A higher BMI has been demonstrated in the Pro12Ala heterozygotes in certain populations like the white Americans, Iranians and other Caucasian populations [10,13,14] while few studies have also demonstrated a lower BMI in the Pro12Ala polymorphic individuals [13,15]. Genetic heterogeneity due to differences in ethnicity could be contributory to the discrepancies [13,16].
A metanalysis in British subjects has shown that the Pro12Ala polymorphism has an apparent effect on BMI only in markedly obese individuals. When BMI of the three genotype groups are compared, it is found that the Ala12 homozygotes have significantly higher BMI than heterozygotes and Pro12 homozygotes indicating that the Ala12 allele is associated with increased BMI, particularly in the obese individuals [17].
The effect on BMI can be ascribed to an increased PPAR γ expression in the adipose tissue of obese subjects, but this expression can be down-regulated by low-calorie diet. It has been shown that, when the dietary polyunsaturated fat to saturated fat ratio is low, the BMI in Ala12 carriers is greater than that in Pro12 homozygotes, but when the dietary ratio is high, the opposite is seen. This gene–nutrient interaction may, in part, explain the differential effects of the polymorphism on the body mass index in obese and lean individuals [18].
In addition, studies in the Indian population have largely shown that there are no differences in BMI, waist-hip ratio and waist circumference between the carriers of the polymorphism and the wild-type genotype in contrast to the Caucasians [10,11]. Contradictorily, a higher BMI has also been observed [12]. However, the BMI of individuals with the Pro12Ala genotype did not significantly differ from individuals with the Pro12Pro genotype in the global analysis implying only a modest effect of the polymorphism [9,17].
The first evidence for an association between the Pro12Ala polymorphism in PPARG gene and increased insulin sensitivity was reported by Debb et al., [15]. A reduced transcriptional activity of the PPARG2 gene as a result of Proline to Alanine aminoacid substitution has been demonstrated but however the mechanism by which the reduced transcriptional activity of PPARγ influences insulin sensitivity is still unclear [15].
Moreover, multiple studies have proven that Pro12 Ala polymorphism reduces the risk of type 2 diabetes mellitus [19–21]. In addition, the progression from normal glucose tolerance to clinical type2 diabetes in which insulin resistance plays a major risk factor, is genetically influenced by this polymorphism [20]. Currently it is also recognized that this polymorphism plays an important role in the risk of nephropathy in diabetes patients [21].
On the contrary, studies have also revealed that reduction in the risk of diabetes is not conferred by the polymorphism in the Indian population unlike that seen in Caucasian population [10,12]. It is interesting to note that some studies also refute such differential effects of the polymorphism ascertaining that the increased insulin sensitivity and protective role of the polymorphism in reducing the risk of diabetes is also evident in the Asian Indians [11,16]. The disparate results are attributed to the diversity in ethnicity of the Indian sub-populations [11].
This is the first study in the South Indian population evaluating the effect of the Pro12Ala polymorphism on therapeutic response to pioglitazone, a PPAR gamma agonist. A statistically significant genetic association between Pro12Ala polymorphism and therapeutic response to pioglitazone [Table/Fig-4] was detected implying a potentially significant contribution of the PPARG2 gene in determining the response to the drug. It has been difficult to explain why paradoxically, both reduced transcriptional activity due to Pro12Ala polymorphism and pharmacological activation of PPARγ by thiazolidinediones result in improved insulin sensitivity. Yamauchi et al., have proposed that the supra-physiological activation of PPAR gamma by thiazolidinediones markedly increases the triglyceride content of white adipose tissue, thereby decreasing triglyceride content of liver and muscle [22]. This in turn leads to amelioration of insulin resistance at the expense of obesity whereas a moderate reduction of PPAR gamma activity by Pro12Ala polymorphism decreases triglyceride content of white adipose tissue, skeletal muscle, and liver due to increased leptin expression and fatty acid combustion along with decreased lipogenesis, thereby ameliorating high fat diet induced obesity and insulin resistance. Thus even though different mechanisms appear to be involved, both act to reduce insulin resistance. This additive effect could be the possible mechanism by which the study participants with Pro12Ala polymorphism have demonstrated a better therapeutic response compared to those with Pro12Pro genotype.
Similar studies done in other populations have yielded conflicting evidence [23–25]. These studies were performed in different ethnic populations like the Chinese, Iranian, and German. A study done in thirty, type 2 diabetes patients in a North Indian population revealed a better glycaemic response of those with Pro12Ala polymorphism to pioglitazone monotherapy [26]. A metanalysis of five studies in which the effect of Pro12Ala polymorphism on the pharmacodynamics of pioglitazone was examined, showed that three studies proved a genotype phenotype correlation while the rest did not reveal such association. According to the metanalysis, this disparity in the results could be attributed to factors like heterogeneity of the patient population in the studies included in the metanalysis, their inclusion/ exclusion criteria, different pioglitazone treatment regimens and presence of other anti-diabetic treatments. Thus PPARG2 gene Pro12Ala polymorphism could be contributory to the inter-individual variability observed in therapeutic response to pioglitazone, even though the variability cannot be completely explained by the polymorphism indicating the possible involvement of other genetic and environmental factors [27].
Conclusion
The current study has demonstrated a significant association between the Pro12Ala polymorphism and a better glycaemic response to pioglitazone. But the major limitation of the study is small sample size. Therefore the results require confirmation in larger trials involving larger sample before establishing the genotyping for Pro12Ala polymorphism as a predictor of response to pioglitazone therapy in diabetes. Thus the study emphasizes that genotyping could, in future, guide in optimizing diabetes management in individual patients and that pharmacogenetics has an important role to play in achieving the goal of individualized therapy in patient care.