Coronary sinus (CS) and great cardiac vein (GCV) are increasingly being used as a conduit for venous catheterisation for performing various cardiac interventions. These include measurement of energy substrate (glucose and fatty acids) concentrations in CS blood [1] and CS temperature, during retrograde cardioplegia [2]. The coronary venous system is also used for left ventricular or biventricular pacing in patients with severe heart failure [3], for ablating posteroseptal accessory neural pathways by radiofrequency energy or cryothermic ablation [4], coronary venous retroperfusion for reduction of infarct size following acute coronary occlusion [5] and for percutaneous transvenous mitral annuloplasty for the treatment of functional mitral regurgitation [6].
The procedure might however become complicated due to obstruction offered by anatomical causes namely, the valve of CS (Thebesian valve) [7], the acute bend of the GCV and valve of Vieussen’s [8]. A displaced CS catheter might lead to various cardiac complications like haemopericardium, myocardial damage and haematoma in the right ventricle [9]. Use of imaging modalities and knowledge of potential variations of the cardiac venous system would allow for anticipation of impediments during interventional procedures [10].
The aim of the present study was to expound the anatomical considerations of coronary venous catheterization and to elucidate the potential causes of obstruction and the complications of the procedure.
Materials and Methods
A cross-sectional observational study was carried out from November 2013 to March 2014 on 40, formalin fixed cadaveric human hearts procured from north Indian adults (Age: 24-65 years), out of which 37 were male and 3 were female. Hearts of adults, who had died of non cardiac causes, were included in the study while those with congenital anomalies, gross pathology or with previous cardiac surgeries were excluded from the study. Coronary sinus and GCV alongwith the accompanying arteries were dissected by removing the epicardium and the subepicardial fat. The wall of the right atrium was incised along the sulcus terminalis to view CS ostium. The length of CS (from its junction with the GCV to coronary sinus ostium in right atrium) and the maximum transverse diameter of the CS ostium were recorded and its course was observed. The length of GCV was measured using a thread placed along its course starting near the apex of the heart till its termination in the coronary sinus. This thread was straightened and its length was measured. The diameter of the GCV was measured near its termination and its course was described. The measurements were done using digital Vernier callipers (Supplier: Effem technologies, New Delhi, India). The angle of bend of GCV was measured using a 360 degree goniometer (Supplier: India Medico Instruments, New Delhi, India). This was done by placing one prong of the goniometer along the vein’s course in the anterior inter-ventricular groove and the other prong along its course in the atrio-ventricular groove [Table/Fig-1]. The relation of the CS and GCV with their neighbouring arteries was described. Thebesian valve was studied with respect to its morphology and the percentage coverage of the coronary sinus ostium. This was calculated by percentage of the transverse diameter of coronary sinus ostium covered by Thebesian valve (maximum length of Thebesian valve/ coronary sinus ostium diameter X 100). The pictures were clicked by a Nikon Coolpix P900s camera. The data was tabulated and mean, range and standard deviation of all quantitative data was calculated using Microsoft Excel 2010 software.
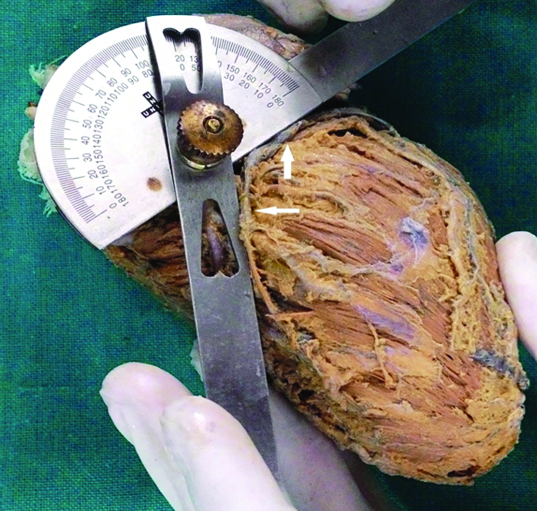
Measurement of the angle of bend of great cardiac vein (marked by white arrows).
Results
Coronary sinus
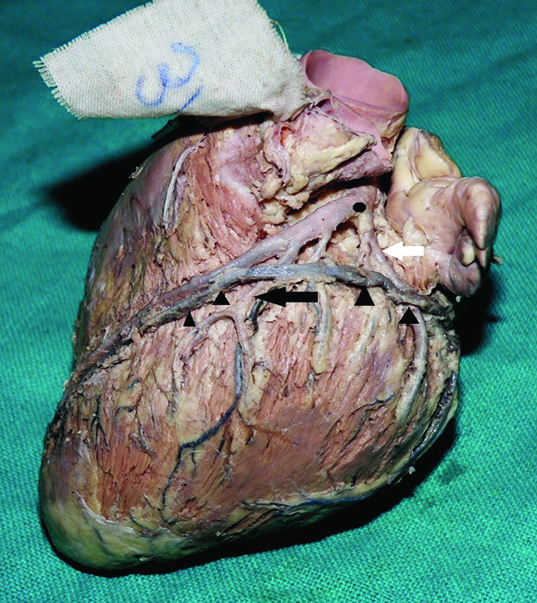
Coronary sinus traversed the left posterior part of the atrio-ventricular groove [Table/Fig-2]. Near its distal end it was observed to be deviated slightly superiorly to be related to the posterior aspect of the wall of left atrium. It opened in the wall of the right atrium, between the tricuspid opening and the opening of inferior vena cava, lying posterosuperior to the commissure between septal and posterior leaflet of the tricuspid valve. In 23 hearts CS crossed ventricular branches of circumflex artery superficially.
Posterior view of the heart showing the course of coronary sinus in the left posterior part of the atrio-ventricular groove. White arrow is pointing at the coronary sinus. White bracket depicting the length of coronary sinus.

The length of CS was in the range of 20.89mm to 39.62mm. The mean length was 35.35mm ±4.43mm (1SD).
The transverse diameter of CS at its ostium was in the range of 7.34mm to 16.52mm. The mean diameter at the ostium was 11.75mm ±2.66mm.
The mean diameter of the coronary sinus ostium in the hearts where Thebesian valve was absent was found to be 16.11±0.56mm, while in those where it was present the mean diameter was found to be 11.40 ±2.44mm.
Great cadiac vein
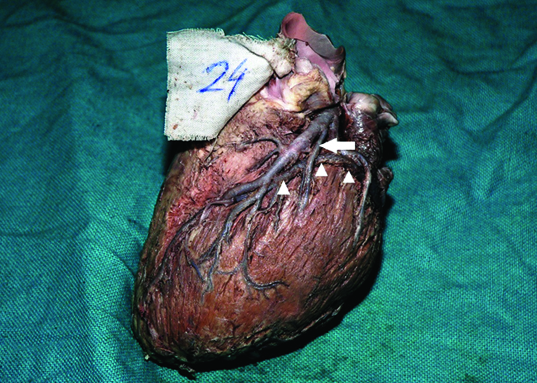
GCV started at a variable distance from the apex of the heart, in the anterior interventricular groove. In the groove, it ran either superficial (12/40 hearts) [Table/Fig-3] or deep (28/40 hearts) to the left diagonal artery [Table/Fig-4]. It then turned at an obtuse angle and continued in the coronary sulcus, forming the base of the triangle of Brocq and Mouchet along with the two branches of left coronary artery, i.e. anterior interventricular and the circumflex artery [Table/Fig-3]. Along its course, it crossed the circumflex artery superficially.
Left view of the hearts showing the great cardiac vein (course marked by black arrowheads) crossing left diagonal artery (black arrow) superficially. Great cardiac vein is seen to form the base of triangle of Brocq and Mouchet with the two terminal branches of left coronary artery. Point of bifurcation of left coronary artery is marked by the black star. Great cardiac vein is seen crossing superficially the circumflex artery (white star) and left marginal artery (white arrow).
Left view of the heart showing the great cardiac vein (white arrowheads) passing deep to the left diagonal artery (white arrow).
The length of the GCV was in the range of 52.38mm to 147.65mm. The mean length was 96.23 ±22.52mm.
The diameter of the GCV near its termination ranged from a minimum of 3.95mm to a maximum of 7.64mm. The mean diameter was 5.99 ±1.02mm.
Angle of the great cardiac vein
The angle of the GCV as it turned left to continue in the left posterior part of the atrio-ventricular groove ranged from a minimum of 92 degrees to a maximum of 122 degrees. The mean angle was 107 ±6.74 degrees.
The besian valve
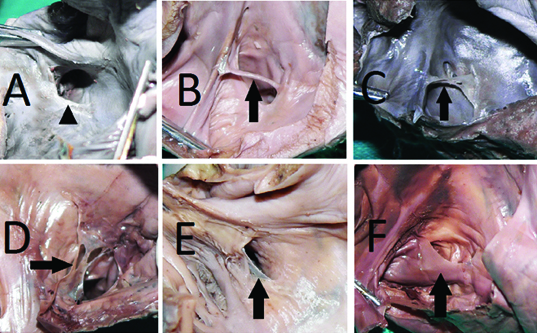
The valve of the coronary sinus was present in 92.5% (37/40) of the hearts. In 7.5% (3/40) hearts, no valve was seen at the ostium [Table/Fig-5a]. Various morphologies of the Thebesian valve were observed [Table/Fig-5b-f,6]. The average percentage coverage of CS ostium was 40%. In five hearts thebesian valve covered more than 50% of CS ostium [Table/Fig-5f].
Interior view of the right atrium showing various morphologies of Thebesian valve (black arrow): a) Absent valve, Arrow pointing at the CS ostium; b) Thin band without fenestration; c) Thin band with fenestrations; d) Broad band with fenestrations; e) Thin semilunar; f) Thick semilunar, covering more than 50% of the CS ostium.
Prevalence of various morphologies of Thebesian valve.
| S. No. | Thebesian Valve Morphology | No. of hearts, Percentage |
|---|
| 1. | Thin Band without fenestrations (Fig 5B) | 3, 7.5% |
| 2. | Thin band with fenestrations (Fig 5C) | 1, 2.5% |
| 3. | Broad band with fenestrations (Fig 5D) | 5, 12.5% |
| 4. | Thin semilunar (Fig 5E) | 14, 35% |
| 5. | Thick semilunar (Fig 5F) | 17, 42.5% |
No statistically significant differences were observed in the quantitative data between hearts from male and female cadavers or between hearts from various age groups.
Discussion
Coronary sinus
The coronary sinus deviated from its location in the left posterior part of the coronary sulcus, to become directly related to the posterior aspect of the left atrium before it opened into the right atrium. As suggested by previous researchers, anatomical and histological connections between inferior right atrium and the left atrial myocardium via a cuff of cardiacmuscle around the CS may be responsible for such a shift [11,12]. A detailed histological study might be helpful to confirm the presence of such fibres and their effect on the course of coronary sinus.
The range of length of CS described in the present study (20.89 – 39.62mm) exceeded the range of 2-3 cm documented in the standard anatomy textbooks [13]. El Massarany in their study on 32 cadaveric human hearts found the length of the coronary sinus to be 48.4± 5.2mm [14]. The mean length of CS (35.35±4.43mm) was less than described by El Maasarany but was similar to the length described by Maros et al., in their study of 54 adult human hearts (36mm) [15]. These differences could be due to inherent genetic differences between the populations studied or due to the lower socioeconomic status of the subjects in the present study.
The diameter of CS ostium is a critical factor determining the success rate of coronary sinus catheterisation. In the past, various researchers have documented the diameter of CS ostium in their studies. Maros et al., reported the mean CS ostium diameter as 9mm in their study on 54 cadaveric human hearts [15]. Christiaens et al., in their 16 row multidetector CT scan study in 50 patients reported the transverse and craniocaudal diameter of the CS ostium to be 12.2+/-3.6 mm and 15.3+/-3.7 mm respectively [16]. The average transverse and craniocaudal dimensions of CS ostium in hearts with Thebesian valves was reported to be significantly shorter than those without Thebesian valve by Mak et al., [17].
In the present study, the mean diameter of CS ostium in the 3 hearts where Thebesian valve was absent was 16.11mm with a standard deviation of 0.56mm, which was more than the diameter in the 37 hearts where the Thebesian valve was present (mean diameter 11.40mm with a standard deviation of 2.44mm). It can thus be inferred that the advancing catheter would not encounter any obstruction at CS ostium in hearts where Thebesian valve is absent owing to a larger ostial diameter in such cases.
A positive correlation has been found between the diameter of CS ostium with various parameters like age of the patient [17], weight of the heart [18] and right atrial end systolic volume [19]. A correlation of CS diameter with anthropometric parameters may help surgeons decide upon the size of the venous catheter to be used in a particular patient without the need of imaging modalities. Future studies may be done to address the correlation of various anthropological parameters with CS ostium diameter.
In the 23 hearts where ventricular branches of circumflex artery pass deep to CS, the former might be compressed during CS catheterisation leading to myocardial ischemia.
Great cadiac vein
The course of the GCV especially in relation to the neighbouring arteries was elucidated in the present study. GCV coursed superficial to the left diagonal artery in 12 (30%) hearts, superficial to left marginal artery in 17 (42.5%) hearts and in all the 40 hearts it crossed the circumflex artery superficially. However, unlike the findings of Gerber et al., who documented a crossover between the GCV and the anterior interventricular artery in 12% patients, in the present study, the GCV in all 40 hearts coursed parallel to the anterior interventricular artery (on its left side) and there was no crossover observed between the two [20]. Similar to our findings, Ortale et al., described the GCV forming the base of the arteriovenous triangle of Brocq and Mouchet with the bifurcating branches of the left coronary artery [21]. Pejkovic, reported that the vein lies superficial to the arteries in the triangle in 61% cases and the triangle is crossed by anterior ventricular branches of the left coronary artery [22]. These findings should be borne in mind during catheterisation as the arteries running deep to GCV are at a risk of compression during the procedure and can lead to myocardial ischemia.
The authors believe that the present study provides the first documentation of the length of GCV (96.23 ±22.52mm) as despite an extensive search of literature, no data could be found pertaining to this parameter. The diameter of GCV in the present study (5.99 ±1.02mm) was similar to that documented by El-Maasarany (5.6+1.6mm) [14].
Angle of the great cardiac vein
Studies by previous researchers have mentioned the course of GCV but none mention the quantification of the angle of its bend from the anterior interventricular to the atrio-ventricular groove [14,22]. Corocoran reported that the bend of GCV was the cause of obstruction to the catheter in 56% hearts after the valve of Vieussens was negotiated. Though they mentioned the word ‘acute’, they did not specify whether they meant an abrupt bend or they referred to the angle of the bend of GCV [8]. In our study, the angle of bend, though abrupt, was always obtuse (107 ±6.74 degrees).
Thebesian valve
In the present study, valve of the coronary sinus was present in 92.5% (37/40) of the hearts. Studies by previous researchers have reported Thebesian valve to be present in 49 to 88% hearts [7,14,23,24]. We observed various morphologies of the valve including a fibrous band (5%), fenestrated (7.5%) and a semilunar fold (80%). The prevalence of a potentially complicating Thebesian valve has been reported to range from 16% to 36% by earlier authors [7,18,23]. The causes of obstruction may be an extensive coverage of the CS ostium or a fibrous, fibromuscular or muscular composition without fenestrations [7]. In the present study, though a well-developed semilunar valve was present in 80% hearts, only in 12.5% hearts it showed an extensive coverage of the CS ostium which can obstruct the passage of the catheter. The Thebesian valve was absent in 7.5% hearts in the present study. The absence might be congenital or might be a result of the force exerted by blood flow across a thin flimsy endocardial valve. When absent, the entry of the catheter through the CS ostium would be uneventful especially as the diameter of the CS ostium was more in hearts without Thebesian valve as compared to hearts where the valve was present. Various imaging modalities have been used to study the coronary venous system morphology. These include CT scan, MRI scan or fibreopticendocardial visualisation catheter [16,17,25].
Conclusion
Thebesian valve may cause obstruction to the catheter due to an extensive coverage of coronary sinus ostium, which is seen in 12.5% cases. The obtuse angle of great cardiac vein has to be negotiated in order to enter this vessel. The information provided in the present study regarding morphology of coronary sinus, great cardiac vein and thebesian valve, if used in conjunction with radiological procedures will help cardiothoracic surgeons and interventional cardiologists to achieve successful catheterisation and to minimise the complications of the procedure including arterial compression leading to myocardial ischemia.