Despite of an increasing body of knowledge and prevention, head trauma (HT) is a major cause of death, disability among young adults and important public health problem in the world especially in developing countries [1–5]. Annually more than 2.4 million referred to hospitals or deaths are related to TBI [6]. In the United States during the years 2006–2010 visits to emergency departments for TBI was about 30% [7]. Approximately 5.3 million Americans are living with disability from TBI and 1.7 million persons have a TBI annually [6,8]. HT led to 52,000 deaths in the USA and 5000 in the United Kingdom a year [9]. The direct and indirect costs of TBI annually are $76.5 billion in the USA [10].
Despite recovery in trauma systems and intensive care the problem of severe TBI remains substantial and mortality rate around 40% have been reported in various study. TBI is a progressive dysfunction that is result to biochemical and metabolic changes and consequently leads to cell death [11]. Evaluating outcome following head injury is essential to reduce complications and improve outcome, clinical equipment utilization and quality of care for head trauma patients. There is no accurate information of outcome and predictive factor following HT in Iran. To the best of our knowledge, because of the limited study of outcome following severe head trauma the aim of this study was to assess the outcome and predicting factor following severe head trauma at the Imam Khomeini Hospital affiliated with the Ilam University of Medical Sciences (west of Iran), during the year 2015.
Materials and Methods
Study Design
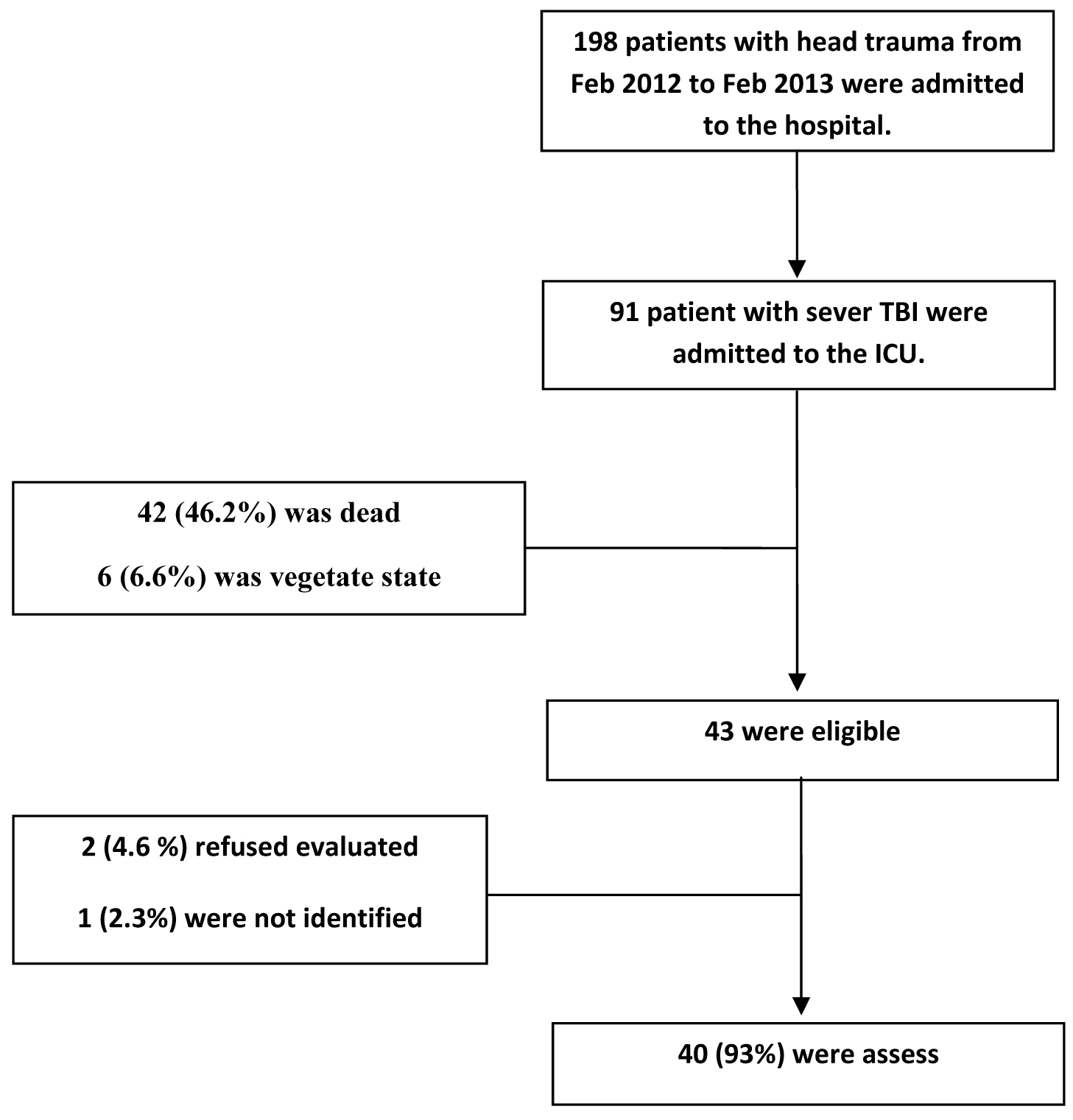
This is a descriptive, retrospective and cross- sectional study that was carried out at the Imam Khomeini Hospital (center of trauma and surgery) affiliated with Ilam University of Medical Sciences (west of Iran), during the year 2015. The population included all patients that to severe head trauma, admitted in the ICU ward from Feb 2012 to Feb 2013 [Table/Fig-1]. In this study we assess the outcome of the physical and psychological function and quality of life after 1 years following severe head trauma. The inclusion criteria included: 18≤ year of age, Glasgow Coma Scale (GCS) score ≤ 8 within the first 48 hour of admission, not having other trauma such as internal haemorrhage or trauma to the chest. Patients with abdominal or orthopedic trauma, gunshot injuries and absence of samples information were excluded. Data were collected from two sections; one section consisting of a questionnaire answered by the patients and other section from the patient records.
Instrument
The questionnaire used in this study was an author-developed scale which adopted from several previous scale in literature review. These scale include Glasgow Outcome Scale (GOS) [12] SF-36 questionnaire and the Hospital Anxiety and Depression Scale (HAD) [13]. The GOS is a multi-dimensional scale which assesses various aspects of outcome. The five categories of the original GOS scale are: Grade (I) dead; Grade (II) vegetative (absence of awareness, cannot interact, unresponsive) and Grade (III) severely disabled (conscious but disabled and dependent); Grade (IV) moderately disabled (disabled but independent) and Grade (V) good recovery (can work). GOS of I-III was considered as unfavourable outcome and GOS of IV, V was considered as favourable outcome for statistical analysis [12].
The SF-36 is a 36-item self-report questionnaire that consist of eight dimensions. Physical functioning (PF), social functioning (SF), physical role (PR), emotional role (ER), mental health (MH), vitality (VT), bodily pain (BP) and general health (GH) [13]. The reliability and validity of the SF-36 have been established in a TBI population and it has been widely used in TBI research [1,12] The Persian SF-36 questionnaire for assessment of QOL was filled. SF-36 scoring is between 0 - 100, higher scores indicate a good QOL. Scores > 60 signify good QOL and scores < 60 suggest unfavourable QOL [5].
The HAD scale was a questionnaire to measure psychological outcome. This instrument used to measure anxiety and symptoms of depression for patients without psychiatric disorders and consistent of 14 items, 7 items with anxiety (HAD-A), and 7 with symptoms of depression (HAD-D). The score ranges between 1 and 21, and scores ≥8 indicate anxiety or symptoms of depression. The validity of the HAD scale have been established in previous study. Cronbach alpha for the HAD-A was 0.83 and for the HAD-D 0.82 [14].
The purposive sampling was used. First by referring to records of the patients during the Feb 2012 to Feb 2013 admission with HT diagnosis, eligible samples were identified. The parameters demographic included age, sex, mode of injury, GCS on admission, hospital and ICU stay were recorded. After visiting the patients, the GOS, SF-36 and HAD scale were completed [Table/Fig-2].
Clinical outcome and characteristics of the patients.
| Parameter | Survivors (n=49) | Non Survivors (n= 42) | p-value |
|---|
| Age (year) (Mean ±SD) | 34.3± 17.2 | 40.2± 19.7 | > 0.05 |
| Length of ICU stay (day) (Mean ±SD) | 20.3± 17.9 | 11.5 ± 14.4 | <0.05 |
| Length of hospital stay (day) (Mean ±SD) | 28.1± 19.7 | 12.4± 16.1 | <0.001 |
| Admission GCS, (Mean ±SD) | 6 ± 1.5 | 5± 2 | <0.05 |
| Mechanical ventilation (n %) | 47(95.9%) | 42 (100%) | > 0.05 |
| VAP & ARDS (n %) | 14 (28.6%) | 6 (14.3%) | < 0.05 |
| Pupil reactivity (n%) |
| Both reactive | 43 (87.7%) | 8 (19%) | <0.05 |
| One reactive | 6 (12.3%) | 11 (26.3%) | <0.05 |
| None reactive | 0.00 | 23 (54.7%) | <0.05 |
| Sex (M/F) (n %) | 44/5 (89.8%/10.2%) | 24/18 (57.1%/42.9%) | < 0.05 |
| Mode of injury (n%) |
| Traffic accidents | 39 (79.6%) | 34 (81%) | >0.05 |
| Fall | 10 (20.4%) | 8 (19%) | >0.05 |
Ethical Consideration
After getting written permission (grant no: 22/1/3180; JUN/27/2015) in addition to co-ordinating with the hospital managers, informed consents were obtained from all the participants.
Statistical Analysis
All statistical analyses were performed using SPSS, version 16 (SPSS Inc, Chicago, IL, USA). Categorical data were expressed as percentages. Values were presented as mean ±SD. One-way ANOVA, independent t-test, Pearson correlation coefficient or Spearman rank order correlation test and logistic regression analyses were performed when appropriate. The p < 0.05 was considered significant.
Results
A total of 873 patients admitted in the trauma ICU ward from Feb 2012 to Feb 2013, 198 patients were having head trauma and 91 patients were severe head trauma (GCS ≤ 8). The mortality rate of the patients was (46.2%), (6.6%) vegetative, (8.8%) sever disability, (4.4%) moderate disability and (34.1%) good recovery. A total of 49 alive patients, 2 (4.6 %) refused evaluated and 1 (2.3%) were not identified.
The QOL of the patients in most dimension were impaired and (58%) of patients had unfavourable QOL. The mean dimensions of QOL was: Physical functioning (62.2 ±15.8), Social functioning (54.4 ±24.6), Role Physical (59.3±35.6), Role Emotional (69.8 ±36.9), Mental health (53.5±23.2), Vitality (55.6±21.8), Bodily pain (55.7±26.4) and General health (58.3±16.2). About (37.5%) of patients with anxiety and (27.5%) had a depression. According to univariate analysis a significant correlation was found between age, GCS arrival, length of ICU stay, mechanical ventilation, VAP & ARDS and pupil reactivity with QOL, GOS, HAD-A and HAD-D (p <0.05, p < 0.001) [Table/Fig-3].
Correlation between different variables with QOL, HAD-A and HAD-D.
| Parameter | PF | SF | RP | RE | MH | VT | BP | GH | GOS | HAD-A | HAD-D |
|---|
| Age | *r= -0.56 | ***r= -0.18 | **r= -0.32 | ***r= -0.13 | ***r= -0.19 | ***r= -0.14 | ***r= -0.17 | **r= -0.29 | **r= -0.46 | **r=0.45 | ***r=0.22 |
| Sex | ***r=0.13 | ***r=0.17 | ***r=0.11 | ***r=0.21 | ***r=0.14 | ***r=0.19 | ***r=0.16 | ***r=0.23 | ***r=0.19 | ***r=0.22 | ***r=0.21 |
| Admission GCS | *r=0.46 | *r=0.68 | **r=0.32 | ***r=0.16 | **r=0.38 | ***r=0.14 | ***r=0.16 | **r=0.36 | *r=0.43 | **r=-0.49 | **r= -0.48 |
| ICU stay | *r= -0.66 | ***r= -0.18 | *r= -0.56 | ***r=-0.14 | ***r= -0.22 | ***r= -0.12 | ***r=-0.19 | ***r=-0.23 | *r= -0.45 | **r=0.36 | **r=0.49 |
| Hospital Stay | ***r= -0.21 | ***r= -0.14 | ***r= -0.23 | ***r=-0.16 | ***r=-0.11 | ***r=-0.14 | ***r=-0.19 | ***r=-0.21 | ***r= -0.16 | ***r=0.13 | ***r=0.23 |
| Mechanical Ventilation | **r= -0.44 | **r= -0.32 | *r= -0.56 | ***r=-0.16 | *r=- 0.38 | **r=-0.36 | ***r=-0.18 | ***r=-0.19 | **r= -0.33 | ***r=0.23 | **r=0.28 |
| VAP & ARDS | **r= -0.31 | **r= -0.36 | *r=- 0.46 | ***r=-0.16 | ***r=-0.13 | ***r=-0.12 | ***r=-0.16 | ***r=-0.21 | **r= -0.36 | ***r=0.19 | ***r=0.18 |
| Pupil Reactivity | **r=0.37 | ***r=0.13 | *r=0.66 | ***r=0.23 | ***r=0.11 | ***r=0.18 | ***r=0.13 | ***r=0.19 | *r=0.46 | ***r=-0.13 | ***r=-0.17 |
| Mode of injury | ***r=0.11 | ***r=0.16 | ***r=0.21 | ***r=0.23 | ***r=0.14 | ***r=0.19 | ***r=0.13 | ***r=0.15 | ***r=0.09 | ***r=0.24 | ***r=0.21 |
*p<0.001, **p< 0.05, ***p>0.05
The logistic regression analysis show that GCS arrival a predicate factor for QOL and GOS (p <0.001, OR : 1.75, 1.94 respectively), length of ICU stay a predicate factor for QOL and GOS (p <0.05, OR : 1.11, 1.28 respectively), mechanical ventilation a predicate factor for GOS (p <0.001, OR : 1.78), VAP & ARDS and pupil reactivity a predicate factor for GOS (p <0.05, OR : 1.36; p<0.001, OR : 1.94 respectively). The GCS arrival and ICU stay a predicate factor for HAD-A (p<0.05, OR: 1.73, 1.38 respectively). The Wald test show that GCS arrival, pupil reactivity, ICU stay, mechanical ventilation and VAP & RDS are the most important variables in prediction respectively [Table/Fig-4,5].
Logistic regression analysis between different variables with QOL and GOS.
| Parameter | QOL | GOS |
|---|
| B | SE | Wald | p | Odds Ratio | B | SE | Wald | p | Odds Ratio |
|---|
| Age | 0.74 | 0.17 | 2.14 | 0.07 | 0.78 | 0.023 | 0.10 | 0.43 | 0.849 | 0.13 |
| Sex | 0.02 | 0.75 | 0.12 | 0.85 | 0.16 | 0.041 | 0.48 | 0.01 | 0.84 | 0.45 |
| Admission GCS | 1.64 | 0.12 | 13.46 | <0.001 | 1.75 | 1.76 | 0.13 | 25.32 | <0.001 | 1.94 |
| ICU stay | - 1.23 | 0.49 | 11.47 | 0.03 | 1.11 | -1.84 | 0.1 | 10.36 | 0.03 | 1.28 |
| Hospital stay | -0.54 | 0.11 | 0.97 | 0.12 | 0.14 | -0.66 | 0.02 | 1.45 | 0.163 | 0.01 |
| Mechanical ventilation | -0.01 | 0.23 | 1.36 | 0.07 | 0.02 | -1.84 | 0.12 | 14.56 | <0.001 | 1.78 |
| VAP & ARDS | -0.41 | 0.34 | 0.96 | 0.09 | 0.12 | -1.63 | 0.03 | 10.34 | 0.04 | 1.36 |
| Pupil reactivity | 0.01 | 0.01 | 1.46 | 0.28 | 0.03 | 1.11 | 0.14 | 17.28 | <0.001 | 1.94 |
| Mode of injury | 0.01 | 0.45 | 0.23 | 0.65 | 0.26 | 0.22 | 0.32 | 0.43 | 0.852 | 0.06 |
Logistic regression analysis between different variables with HAD-A and HAD-D.
| Parameter | HAD-A | HAD-D |
|---|
| B | SE | Wald | p | Odds Ratio | B | SE | Wald | p | Odds Ratio |
|---|
| Age | 0.64 | 0.12 | 1.14 | 0.17 | 0.01 | 0.01 | 0.014 | 0.13 | 0.04 | 0.20 |
| Sex | 0.04 | 0.13 | 0.02 | 0.89 | 0.04 | 0.13 | 0.189 | 0.21 | 0.64 | 0.03 |
| Admission GCS | -1.16 | 0.12 | 14.46 | 0.03 | 1.73 | -0.76 | 0.026 | 1.42 | 0.58 | 0.03 |
| ICU stay | -1.12 | 0.36 | 9.47 | 0.05 | 1.38 | 0.94 | 0.143 | 1.36 | 0.09 | 0.07 |
| Hospital stay | 0.14 | 0.03 | 0.27 | 0.62 | 0.03 | 0.36 | 0.121 | 0.14 | 0.66 | 0.02 |
| Mechanical ventilation | 0.03 | 0.06 | 0.36 | 0.17 | 0.01 | 1.04 | 0.027 | 9.14 | 0.05 | 0.86 |
| VAP & ARDS | 0.21 | 0.12 | 0.36 | 0.99 | 0.02 | 0.62 | 0.032 | 0.03 | 0.94 | 0.01 |
| Pupil reactivity | -0.02 | 0.01 | 0.04 | 0.78 | 0.01 | -0.10 | 0.113 | 0.02 | 0.83 | 0.12 |
| Mode of injury | 0.02 | 0.05 | 0.10 | 0.65 | 0.14 | 0.03 | 0.121 | 0.03 | 0.96 | 0.01 |
Discussion
Severe TBI is a major challenging problem in neurosurgery care. Severe TBI defined as head injury associated with a GCS score of 3 to 8 at 6 hour after injury or deterioration to GCS of 8 or less within 48 hour of injury and lasting for at least 6 hour [2].
Our result show that male gender, young adult and traffic accidents in the HT patients are more evident that is consistent with previous study [15,16]. This finding supports gender-related differences in outcome after HT and the hormonal influence in injured brain. Women are less likely than men to develop complications which are due to both a harms effect of testosterone and a beneficial effect of female sex hormones estrogens [17]. The mortality rate in this study was 46.2%, which this rate was 35.1% in Australia, 32% in Northern Ireland, 12% in Taiwan and 29% in Tunisia [18,19]. In this study the QOL of the patients in most dimension were impaired and most of the patients had unfavourable QOL. Patients who have experienced HT will have a different QOL than the general population due to residual impairments from this type of injury [20]. Settervall et al., conclude that participant one year following severe HT had the high scores in the social, emotional and general health status. The lowest scores were observed in the physical, pain and vitality aspects respectively [20]. In a qualitative study patients complained of changes in physical appearance due to scars or overweight, communication difficulties, loss of direction in life, loss of the conditions they had before the trauma and negative reactions in social interactions [21].
Ringdal et al., found patients with head injuries had lower scores in social functioning and mental health. This finding is consistent with our result and previous research which had shown that severe head injury could be a predictor of poor QoL in such patients [14]. MacKenzie et al., in the United States in relation to the differences in SF-36 domains between patients with different HT severities concluded that patients with injuries scored as 5 or 6 had lower scores on the SF-36 domains than patients with injuries scored equal to or lower than [22]. In the study by MacKenzie et al., on patients with different HT severities, in relation to the differences in SF-36 aspects was observed that patients with GCS score of 5 or 6 had lower scores on the SF-36 aspects than patients with GCS score equal or lower [22].
About half of patients in this study have anxiety and depression. Studies show a high level of psychological disabilities after trauma in ICU patients [23,24]. In addition to, HT was associated with a higher level of anxiety, and psychological distress [14]. Fann et al., in patients with HT found 26% had major depression, and 24% had anxiety disorder.
The most of previous research have shown that arrival GCS is a reliable predictor of final outcome [25,26]. In our study, worse outcome was significantly increasing with decreasing GCS. GCS was an independent risk factor that predicts mortality in the ICU trauma patients. Giacino and Kazanis concluded that GCS score in the first 24 hours was critical in predicting vital signs and functional results during the 2-3 day after the injury [4]. Studies has also show that impaired papillary reactivity have a good documented correlation with unfavourable outcome, duration of mechanical ventilation and length of stay [14] have an impact on the patients’ outcome and QoL after an ICU stay [14,27] that is consistent with this study.
TBI consists of two separate forms: primary and secondary brain injury. The primary brain injury regarding the damage caused by the impact accident, leads to skull fracture, vascular or parenchymal damages and concussion. The primary brain injury consequently led to increase in brain bleeding and increased intracranial pressure. The secondary brain injury that occurred after hours and days following primary brain injury is the result of a complex process. The secondary injury occurred by different factors such as hypoglycaemia, hyperglycaemia, hypoxia, hypotension and anaemia. Complication due to secondary brain injury included haematomas, vasospasm, hydrocephalus, cerebral edema, intracranial hypertension, infection, and seizures [28]. In this study a large group of ICU trauma patients were evaluated. On the other hand data were collected from Level 1 trauma center that provide the highest level of surgical care which this strength point of this study.
Limitations
We recognize some limitations of the study. First: the lack of administrative database of patient’s record. Second: retrospective design of studies. Third: data collected from single center because it is single trauma center which this subject can lead to bias.
Conclusion
To conclude, according to mortality rate in this study, impair in all dimension of QOL and significant correlation between different variables with QOL, HAD-A and HAD-D, the following points is necessary: develop in pre hospital, medical and surgical care for the decrease in mortality rates of HT; the use of trauma triage tools; strict enforcement of traffic rules. Experienced medical personnel, facilities and adequate equipment and standard protocols can improve outcome in HT patients.
*p<0.001, **p< 0.05, ***p>0.05