Synchronous Appearance of Adenocarcinoma and Gastrointestinal Stromal Tumour (GIST) of the Stomach: A Case Report
Ramesh Babu Telugu1, Magesh Pushparaj2, Dipti Masih3, Anna Pulimood4
1 Assistant Professor, Department of General Pathology, Christian Medical College, Vellore, Tamilnadu, India.
2 PG Registrar, Department of General Pathology, Christian Medical College, Vellore, Tamilnadu, India.
3 Associate Professor, Department of General Pathology, Christian Medical College, Vellore, Tamilnadu, India.
4 Professor, Department of General Pathology, Christian Medical College, Vellore, Tamilnadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Ramesh Babu Telugu, Assistant Professor, Department of General Pathology, Christian Medical College, Vellore, Tamilnadu -632004, India.
E-mail: dr.rameshtelugu@gmail.com
Adenocarcinoma is the most common histological type of gastric tumour, accounting for approximately 95% of all gastric carcinomas. Gastrointestinal stromal tumours (GISTs) are rare mesenchymal neoplasms of the digestive tract. Synchronous adenocarcinoma and gastrointestinal stromal tumour (GIST) occurring in the stomach is rare and very few cases have been reported in literature. Synchronous tumours in the stomach are rarely diagnosed preoperatively. A 63-year-old gentleman was diagnosed with a gastric adenocarcinoma on endoscopic biopsy and underwent surgery. Postoperative histopathologic examination revealed 2 synchronous tumours with both adenocarcinoma and GIST. The adenocarcinoma was determined to be the aggressive tumour based on histologic features. GIST was categorized as a very low risk of malignancy, based on its size and mitosis. The patient underwent chemotherapy for adenocarcinoma. He is under follow up and is currently disease free. Careful histopathologic evaluation is required to detect co-existing rare synchronous tumours. Presence of the second tumour may require additional procedures or protocols.
Carcinoma, Gastric, Mesenchymal neoplasm
Case Report
A 63-year-old gentleman was evaluated for his complaints of belching, dysphagia and upper epigastric pain. Gastroscopy revealed a proliferative growth extending from 38cm, involving the whole circumference and extending 4-5cm beyond the gastro-oesophageal junction into the fundus along the greater curvature with surrounding erythematous mucosa. A mucosal biopsy from the growth was reported as moderate to poorly differentiated adenocarcinoma. Abdominal Computed Tomography (CT) revealed circumferential thickening involving the gastro-oesophageal junction causing significant luminal narrowing with extension into the cardia, fundus and along the greater curvature of the proximal body of stomach. Staging laparoscopy showed a very tiny serosal nodule along the proximal lesser curve of stomach. The patient was diagnosed to have locally advanced carcinoma of the gastro-oesophageal junction and was planned for 3 cycles of neoadjuvant chemotherapy followed by reassessment for surgery. Post chemotherapy CT scan showed features consistent with mild disease regression. He underwent an Ivor Lewis oesophagectomy. The oesophagogastrectomy specimen was sent for histopathological examination to the Department of Pathology.
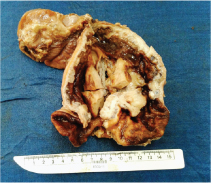
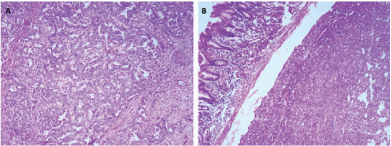
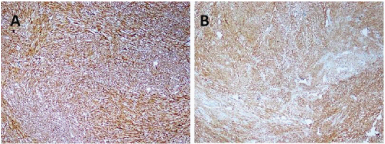
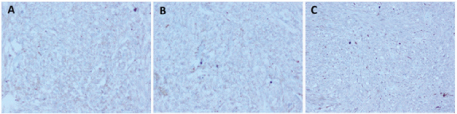
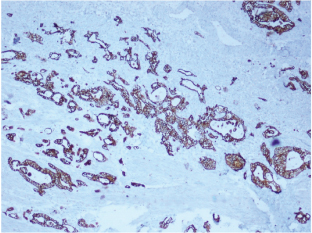
Gross examination of the specimen revealed a proliferative growth (4x3 cm) at the gastroesophageal junction and a tiny submucosal nodule (1x1 cm) with normal overlying mucosa in the fundus of the stomach adjacent to the growth [Table/Fig-1] which was not connected to the gastric cancer. The submucosal nodule was all embedded. Microscopically, the gastroesophageal growth revealed moderately differentiated adenocarcinoma and the submucosal nodule revealed a stromal tumour composed of whorls and short interlacing fascicles of bland spindle cells with eosinophilic cytoplasm consistent with GIST involving the fundus of stomach [Table/Fig-2]. There was no transition between these two components. Adenocarcinoma extended into subserosa with metastatic tumour deposits in 2 out of 4 perigastric lymphnodes (ypT3N1). GIST was mainly located in the submucosa with upto 3 mitoses per 50 high-power fields (pT1). The GIST was of very low risk category based on size and mitosis according to Fletcher’s classification [1]. On immunohistochemistry, the tumour cells of the GIST were diffusely and strongly positive for DOG-1 and CD34 [Table/Fig-3]; negative for CD117, S-100 and smooth muscle actin (SMA) [Table/Fig-4]. The tumour cells of the adenocarcinoma were positive for pancytokeratin [Table/Fig-5]. The patient was treated with EOX regimen (Epirubicin, Oxaliplatin and Capecitabine) chemotherapy for the adenocarcinoma. Post-chemotherapy gastroscopy showed an ulcer and mucosal oedema at the site of the original tumour. Biopsy from the ulcer showed no evidence of malignancy. He is under follow up and is disease free 7 months after surgery.
Gross photograph of oesophagogastrectomy specimen with proliferative growth at gastroesophageal junction.
Photomicrograph of (A) adenocarcinoma and (B) submucosal gastrointestinal stromal tumour (H&E, x100).
Photomicrograph showing diffuse and strong immunohistochemical stains of (A) DOG1 and (B) CD34 (IHCx40).
Photomicrograph showing negative immunohistochemical stains of (A) CD117, (B) SMA and (C) S-100 (IHCx40)
Photomicrograph showing pancytokeratin staining tumour cells of adenocarcinoma (IHCx40).
Discussion
The co-existence of two histologically different neoplasms in the same site is extremely rare. Adenocarcinoma (95%) is the most common malignant tumour of the stomach [2]. Gastrointestinal stromal tumours (GISTs) are common stromal tumours of the digestive tract arising from the interstitial cell of Cajal and account for <1% of all gastrointestinal malignancies [3]. Synchronous tumours are uncommon entities and are usually found incidentally during histopathological evaluation. The incidence of synchronous gastric adenocarcinoma with GIST is about 0.25% [2]. According to literature, co-existence of GISTs with the other tumours ranges from 4.5% to 33%. Stomach is the most common site for GIST. Adenocarcinoma is encountered most frequently as synchronous to GIST in the stomach [4,5]. GISTs have been reported in the gastrointestinal tract from oesophagus to the anus. The most common sites are the stomach (60%), jejunum and ileum (30%), duodenum (5%), and colorectum (<5%) while a few cases (<1%) have been reported in the oesophagus and appendix [6].
Literature search revealed, GISTs co-exist with various tumours like adenocarcinoma, lymphoma, leukaemia, breast carcinoma, prostate carcinoma, pancreatic carcinoma, lung carcinoma or adrenal adenoma [7]. Maionara et al., reviewed a series of six cases of synchronous occurrence of stromal tumours with gastric adenocarcinoma in five patients and one with carcinoid tumour [8]. In our case, the co-existing tumours were located in different sections of the stomach similar to the most of the reported cases [6–8]. Cases of collision tumours with GISTs have also been reported [9]. Synchronous tumours have to be differentiated from collision tumours and composite tumours because the treatment modalities of these tumours differ.
In the present case, GIST was of very low risk category with spindle cells without any pleomorphism, atypia or necrosis. Immunohistochemical staining for DOG-1 and CD34 was strongly and diffusely positive; negative for CD117, S-100 and smooth muscle actin (SMA). The tumour cells of the adenocarcinoma were positive for cytokeratin. Many studies emphasized the expression of CD117 and CD 34 in GIST [10]. According to literature, CD117 is expressed in 80 to 95% of GISTs and only 5 to 20% of cases were negative. A possible histological diagnosis of GIST could be made, only if the tumour is positive for CD34 and negative for CD117. Definite diagnosis is not possible, if the tumour is negative for CD117, CD34, SMA and S100. In such cases, a novel marker, discovered on GIST-1 (DOG1) is useful for the diagnosis of GISTs. DOG1 is a membrane calcium dependent chloride channel expressed specifically and strongly in GISTs with cytoplasmic and membrane staining [11,12]. In our case, definite diagnosis of GIST was possible because the tumour cells were diffusely and strongly positive for DOG1 and CD34; negative for CD117, SMA and S100. Hwang DG et al., performed DOG1 and CD117 immunostain on GIST cell blocks and concluded that all the cases of GIST were positive for DOG1 with strong staining in 90% of cases [13]. CD117 was completely negative in 12% and weakly positive in another 13.5% of GIST cell blocks. The sensitivity and specificity for DOG1 was 100% and 98%, whereas for CD117 was 88% and 100%. Hence DOG1 was considered more sensitive marker than CD117 for the diagnosis of GIST. In another study performed by Yamamoto et al., DOG1 showed 90% immunoreactivity in CD117 negative GISTs [14].
In the present case, owing to the absence of nuclear atypia, necrosis and increased mitotic activity in the GIST, the adenocarcinoma was considered to have greater prognostic significance than the GIST. Surgery remains the cornerstone of the treatment for all resectable nonlymphoma tumours of the stomach. Patients treated for synchronous tumour should receive adjuvant therapy for the more advanced or aggressive tumour type [15]. Our patient was treated with EOX regimen (Epirubicin, Oxaliplatin and Capecitabine) chemotherapy for advanced gastric adenocarcinoma following surgery.
Conclusion
Synchronous tumours are rare neoplasms of the stomach with the co-existence of two histologically different neoplasms occurring in the same site without transition or contact between them. This condition is rarely diagnosed preoperatively. Careful histopathologic evaluation with multiple biopsies from different sites on endoscopy and careful macroscopic and microscopic evaluation of resection specimens is required to detect concomitant tumours. The tumour with advanced or aggressive behaviour has greater prognostic significance and should receive adjuvant therapy.
[1]. Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Diagnosis of gastrointestinal stromal tumours: A consensus approachHum Pathol 2002 33:459-65. [Google Scholar]
[2]. Go JH, Collision of adenocarcinoma and schwannoma of the stomach: a case reportKorean J Pathol 2012 46:373-76. [Google Scholar]
[3]. Nowain A, Bhakta H, Pais S, Kanel G, Verma S, Gastrointestinal stromal tumours: clinical profile, pathogenesis, treatment strategies and prognosisJ Gastroenterol Hepatol 2005 20:818-24. [Google Scholar]
[4]. Agaimy A, Wünsch PH, Sobin LH, Lasota J, Miettinen M, Occurrence of other malignancies in patients with gastrointestinal stromal tumoursSemin Diagn Pathol 2006 23:120-29. [Google Scholar]
[5]. Liszka L, Zielinska-Pajak E, Pajak J, Golka D, Huszno J, Co-existence of gastrointestinal stromal tumours with other neoplasmsJ Gastroenterol 2007 42:641-49. [Google Scholar]
[6]. Villias C, Gourgiotis S, Veloudis G, Sampaziotis D, Moreas H, Synchronous early gastric cancer and gastrointestinal stromal tumour in the stomach of a patient with idiopathic thrombocytopenic purpuraJ Dig Dis 2008 9:104-07. [Google Scholar]
[7]. Samaras VD, Synchronous well differentiated neuroendocrine tumour and gastrointestinal stromal tumour of the stomach: a case reportBMC Gastroenterol 2011 11:1-5. [Google Scholar]
[8]. Maiorana A, Fante R, Maria Cesinaro A, Adriana Fano R, Synchronous occurrence of epithelial and stromal tumours in the stomach: a report of 6 casesArch Pathol Lab Med 2000 124:682-86. [Google Scholar]
[9]. Liu SW, Chen GH, Hsieh PP, Collision tumour of the stomach: a case report of mixed gastrointestinal stromal tumour and adenocarcinomaJ Clin Gastroenterol 2002 35:332-34. [Google Scholar]
[10]. Hasegawa T, Matsuno Y, Shimoda T, Hirohashi S, Gastrointestinal stromal tumour: Consistent CD117 immunostaining for diagnosis, and prognostic classification based on tumour size and MIB1gradeHum Pathol 2002 33:669-76. [Google Scholar]
[11]. Wada T, Tanabe S, Ishido K, Higuchi K, Sasaki T, Katada C, DOG1 is useful for diagnosis of KIT-negative gastrointestinal stromal tumour of stomachWorld J Gastroenterol 2013 19:9133-36. [Google Scholar]
[12]. Simon S, Grabellus F, Ferrera L, Galietta L, Schwindenhammer B, Mühlenberg T, DOG1 regulates growth and IGFBP5 in gastrointestinal stromal tumoursCancer Res 2013 73:3661-70. [Google Scholar]
[13]. Hwang DG, Qian X, Hornick JL, DOG1 antibody is a highly sensitive and specific marker for gastrointestinal stromal tumours in cytology cell blocksAm J Clin Pathol 2011 135:448-53. [Google Scholar]
[14]. Yamamoto H, Kojima A, Nagata S, Tomita Y, Takahashi S, Oda Y, KIT-negative gastrointestinal stromal tumour of the abdominal soft tissue: a clinicopathologic and genetic study of 10 casesAm J Surg Pathol 2011 35:1287-95. [Google Scholar]
[15]. Salemis NS, Gourgiotis S, Tsiambas E, Karameris A, Tsohataridis E, Synchronous occurrence of advanced adenocarcinoma with a stromal tumour in the stomach: a case reportJ Gastrointestin Liver Dis 2008 17:213-15. [Google Scholar]