Beta-thalassaemia major is an inherited, autosomal disorder due to point mutation or, in very rare cases, beta-globin gene deletion on chromosome 11, which leads to decrease in or lack of beta-globin sequence. Ineffective erythropoiesis in beta-thalassaemia is due to defective synthesis of haemoglobin and causes turnover increase in red blood cells (RBCs). A 2-3 units of blood are transfused per 4-6 weeks based on the patient’s condition and the ability to maintain a blood haemoglobin concentration over 10. Iron chelation therapy is usually commenced after 10-15 transfusion turns and through control of ferritin level, and the most common method is subcutaneous infusion of desferrioxamine. The complications due to iron overload include endocrine complications, developmental disorders, deficiency in sexual puberty, diabetes mellitus, etc. In addition, heart disease due to iron overload is the most prevalent cause of mortality in the patients. Treatment by a regular program of blood transfusion and iron chelation leads to decrease in iron overload and increase in life expectancy. However, treatment complications are still high and affect the quality of life [1]. Thalassaemia has been reported as the most common single gene disorder in Iran with the prevalence up to 10% [2].
Immunosenescence is considered as a complex process that negatively affects the development of the immune system and lymphocytes’ function. This process occurs naturally as age advances, but could develop early in young people due to a variety of chronic inflammations, infections, and neoplastic conditions [3]. Oxidative damage is a main factor of accelerating immunosenescence. In fact, stress results in early ageing because of damaging DNA and hence shortening telomeres [4]. Several findings confirm the presence of oxidative condition in the patients with beta-thalassaemia [5], which could lead to early immunosenescence. Regular blood transfusion causes immunosenescence to be accelerated via immuno-suppression and transmission of viruses with immunosuppressive characteristics like cytomegalovirus, hepatitis C virus and Epstein-Barr virus [6].
Continuous antigenic stimulation could decline the cells’ ability to transcribe and turn them into aged cells. Repeated blood transfusion and haemolysis could be the reasons for producing free radicals and oxidative conditions [5]. Splenectomy in addition to immune paralysis against encapsulated bacteria brings about some changes in lymphocytes [7]. This alteration leads to exacerbating the effects of repeated blood transfusion on immune system. Immunosenescence is accompanied with a pronounced decline in naïve lymphocytes’ population which is caused by construction decline in T cells. T cell-mediated immunity depends on the predominance of paticular subsets. One of the most common signs of age-related changes is accumulation of CD28-CD8+ T cells, which could be due to continuous antigenic stimulation of CD28+CD8+T cells. In fact, clonal expansion of CD28- lymphocytes seems to be responsible for increase in infection and failure to respond to vaccines [8].
The available data ragarding the CD+CD28-CD57+ T cell subsets and immunoscenescence are inconsistent. Expression of CD57 on T cells and NK cells is supposed to be a marker of proliferation inability and short telomeres [9]. While many studies have demonstrated that CD28- and CD57+ T cells lose their proliferative capacity, some others have found that these cells can upregulate this capacity under certain stimulation conditions [10].
Gradual decline in responsiveness to antigens is due to immunosenescence and T lymphocytes ageing, and resulted abnormal function leads to T cell-associated immunosuppression. T cells immunosenescence has been confirmed by some characteristics such as failure of CD27 gene expression, telomeres shortening, failure of proliferation, resistance to apoptosis and selective decrease in pro-inflammatory receptors [11,12].
CCR7 is a chemokine receptor that controls homing to lymph nodes and divides memory T cells into two subsets. CCR7+ memory T cells have no immediate effector function, but stimulate dendritic cells efficiently and differentiate to CCR7- T cells after secondary stimulation. CCR7- T cells have exhibited immediate effector function [13].
The infection in thalassaemia patients is prevalent and one of the reasons for death in most patients. The reason for this increased predisposition is not clear in some cases. Since recent studies have indicated immunosuppression and some dimensions of early immunosenescence in the patients with beta-thalassaemia major [5,14,15], and stupendous treatment costs are imposed on the patients with this disease, the present study was conducted to investigate new concepts of early immunosenescence in the patients with beta-thalassaemia major and to identify immunologic complications relevant to the disease and its treatment to develop approaches to prevention of immunologic disorders and to increase life expectancy in these patients.
Materials and Methods
Patients and Controls
This case-control, cross-sectional study was conducted on 27 patients with beta-thalassaemia major referring Hajar Hospital of Shahrekord, Iran between October 2012 and December 2013 with mean age of 17.07±5.02 (range: 10-30) years. Of the patients, 63% were male. A control group of 26 healthy subjects (with mean age of 19.6 ± 6.1 years) matched by age, of whom 57.7% were male, were recruited from local volunteers. Patients entered into the study if they had diagnostic criteria of beta-thalassemia major and were 10-30 years old. The exclusion criteria were hepatitis B and C and HIV positivity, chelation therapy except desferrioxamine, infection within the past three weeks, pregnancy and taking vitamin C or folic acid within the past 48 hours. A checklist containing patients’ information consisting of age, gender, records number, the time of disease diagnosis, total number of blood transfusion, transfusion intervals, volume of transfused blood in each referring, haemoglobin and ferritin levels, splenecotmy, and treatment with desferrioxamine was filled out. After informed consent was obtained, venous blood was collected in three tubes containing EDTA, sodium heparin, and no anti-coagulant.
Measurement of Hematologic Indices and Ferritin
Blood count was measured by EDTA blood sample and automated cell counter (Sysmex KX21, Sysmex Corporation, Japan). Serum samples were separated and ferritin level was measured by ELISA (Orgentec Kit, Germany).
Investigation of Biomarkers CD57, CD28, CD27 and CCR7
The phenotyping was performed with sodium heparin containing blood, and RBCs were lysed by ammonium chloride buffer. Then, the cells were kept in complete culture media. The cells were incubated with directly conjugated monochlonal antibodies in 4°C and darkness. Each combination included anti-CD3PE, anti-CD4APC or anti-CD8 PE-CY5 and one of the phenotyping antibodies; anti-CD27, anti-CD28, anti-CD57 and anti-CCR7 FITC. After incubation, the cells were washed, fixed in paraformaldehyde, and kept at 4°C and darkness until flow cytometric analysis.
Flow Cytometry
Flow cytometric analysis was performed on a flow cytometer (PARTEC, Germany). 2×104 - 5×104 cells were collected, and lymphocytes were gated by FSC versus SSC, and then CD3+ cells were gated versus SSC. Data were analysed using flowmax 2.4 software and displayed as dot plots of CD4 or CD8 versus other phenotyping markers. The expression of each individual marker was analysed versus CD4 or CD8. The absolute number was calculated by flow cytometric data and the cell counts.
Monoclonal Antibodies
The following monoclonal antibodies were purchased from Beckton-Dickinson Immunocytometry System, USA and used in the study: Anti-CD57FITC, anti-CD3PE, anti-CD8PE-Cy5, anti-CD4APC, anti-CCR7FITC, anti-CD28FITC, anti-CD27FITC and mouse IgG1 and IgG2a.
Statistical Analysis
The data on complete blood count, flow cytometric data, and the data obtained from other laboratory tests and clinical findings were analysed by Mann-Whitney and Spearman’s correlation coefficient test in SPSS 11.5.
Results
Considerable Lymphocytosis, but Lower T cell Count in Beta-thalassaemia Major
Transfusion interval was 3.2 ± 0.3 weeks, serum ferritin 1529.3 ± 954.8 ng/ml, and desferal infusion level 5.2 ± 0.8 nights per week.
White blood cell count was higher in the patients (8723.5 ± 8594.10 / mm3) than the control group (5134.13 ± 818.98 / mm3), with no statistically significant difference.
A notable lymphocytosis was observed in the patients compared to the control group (p < 0.001). On the other hand, the proportion of T lymphocytes in the patients (28.63 ± 24.02%) decreased significantly compared to the control group (54.81 ± 14.87%) (p < 0.001). However, the number of T lymphocytes did not change significantly in the patients compared to the control group. The proportion and absolute number of CD4+ and CD8+ T cells in the patients did not change significantly compared to the control [Table/Fig-1].
Immunologic parameters in the patients compared to the control.
| Parameters | Patients (27) | Controls (26) | p-value |
|---|
| Mean±SD |
|---|
| WBC count/mm3 | 8723.5 ±8594.10 | 5134.13±818.89 | NS |
| Lymphocyte count/mm3 | 7984.22 4±620 | 2122.21±543.7 | < 0.001 |
| CD3+ T cells (%) | 28.63±24.02 | 54.8±14.87 | < 0.001 |
| CD3+ T cells count/mm3 | 992.29±582.81 | 1216.29±458.33 | NS |
| CD4+ T cell/mm3 | 576.73±45.13 | 644.15±294.5 | NS |
| CD8+ T cell/mm3 | 405.35±201.17 | 417.04±155.44 | NS |
NS: Not significant
Increased Lymphocyte Count and Decreased T cell Count in Splenectomized Patients
As shown in [Table/Fig-2], a considerable lymphocytosis was observed in the splenectomized group (10674.2 ± 10403.5 /mm3) compared to the unsplenctomized (2747 ± 994.96/mm3) (p < 0.001). However, CD3+ T cells count and frequency were higher in the unsplenectomized patients.
Subpopulations of lymphocytes in splenectomized patients with beta-thalassaemia major compared to the unspelenectomized.
| Parameters | Splenectomized (12) | un-splenectomized (15) | p-value |
|---|
| Mean±SD | |
|---|
| WBC count/mm3 | 13609±11499.4 | 4626.8±1011 | < 0.001 |
| Lymphocyte (%) | 74.41±13.07 | 59.48±16.76 | 0.019 |
| Lymphocyte count/mm3 | 10674.3 ± 10403 | 2747.5±994.96 | < 0.001 |
| CD3+cells /mm3 | 828.3±676.01 | 1123.4±465.86 | 0.043 |
| CD3+cells (%) | 9.91±9.36 | 43.66±21.39 | < 0.001 |
| CD4+ T cell/mm3 | 513.8±378.1 | 648.8±310.4 | NS |
NS: Not significant
Altered Expression of CD27, CD57, and CCR7 on Patients T cells
The frequency of T cells expressing each phenotyping marker was investigated [Table/Fig-3].
Expression of phenotypic markers on peripheral blood CD4+ T cells of a representative sample from beta-thalassaemia patients and healthy controls; the expression of each individual marker was analysed versus CD4. The percentages represent the proportion of CD4+ T cells expressing each phenotypic marker.

There was a tendency toward decreasing the number of CD3+CD4+CD27+ T cells in the patients (p=0.07). The number of CD3+CD8+CD27+ lymphocytes in the patients was not significantly different from that in the control group [Table/Fig-4].
Comparison of the frequency of cells expressing each one of phenotypic markers on CD4+ and CD8+ T cells between the control group and the patients.
| Parameters | patients | controls | p-value |
|---|
| mean±SD | |
|---|
| CD4+ T cells |
| CD27 | 275.71±196.45 | 476.95±255.33 | 0.079 |
| CD28 | 314.53±197.33 | 477.64±269.65 | NS |
| CD57 | 260.64±168.26 | 117.27±67.28 | 0.009 |
| CCR7 | 141.08±96.72 | 306.40±232.33 | 0.027 |
| CD8+ T cells |
| CD27 | 236.83±160.69 | 298.68±119.34 | NS |
| CD28 | 181.22±112.30 | 256.93±123.96 | NS |
| CD57 | 204.14±160.50 | 83.15±64.01 | 0.059 |
| CCR7 | 273.69 ±203.23 | 156.22±102/04 | NS |
No significant difference was observed in the number of CD3+CD4+CD28+ and CD3+CD8 +CD28+ between the patients and the control group. The number of CD3+CD4+CD57+ in the patients was significantly higher than that in the control group, and the number of CD3+CD8+CD57+ cells was relatively higher compared to the control group. The number of CD3+CD4+CCR7+ T cells in the patients was significantly lower compared to the control but in the CD8 subgroup, no significant difference was observed.
Regarding the significant decrease in the number of lymphocytes in the patients compared to the control, we investigated the effect of age, ferritin, blood transfusion, and desferal infusion level on the number of T lymphocytes; the changes in none of them were significantly correlated with the number of T cells.
A Correlation between Ferritin Level and T cell Subtypes
The effect of age, ferritin level, the amount of blood transfusion, and desferal infusion level on the number of the cells representing each one of the investigated lymphocyte markers was also examined [Table/Fig-5].
Correlation of the frequency of T lymphocytes expressing each phenotypic marker with the patients’ age, ferritin level, desferal infusion, and the number of blood units consumed by the patients.
| Type of lymphocyte | | Age | Ferritin level | Transfusion level | Desferal consumption |
|---|
| CD3+ |
| Correlation coefficient | -0.27 | 0.14 | -0.29 | -0.11 |
| p-value | NS | NS | NS | NS |
| CD3+CD4+CD27+ |
| Correlation coefficient | -0.22 | -0.38 | -0.11 | -0.16 |
| p-value | NS | NS | NS | NS |
| CD3+CD8+CD27+ |
| Correlation coefficient | -0.44 | -0.75 | -0.62 | -0.25 |
| p-value | NS | 0.01 | 0.04 | NS |
| CD3+CD4+CD57+ |
| Correlation coefficient | 0.014 | 0.85 | 0.018 | 0.35 |
| p-value | NS | 0.002 | NS | NS |
| CD3+CD8+CD57+ |
| Correlation coefficient | -0.25 | 0.75 | -0.16 | 0.45 |
| p-value | NS | 0.003 | NS | NS |
| CD3+CD4+CCR7+ |
| Correlation coefficient | -0.053 | 0.31 | 0.091 | 0.21 |
| p-value | NS | NS | NS | NS |
| CD3+CD8+CCR7+ |
| Correlation coefficient | - 0.35 | - 0.70 | -0.014 | 0.26 |
| p-value | NS | 0.025 | NS | NS |
| CD3+CD4+CD28+ |
| Correlation coefficient | -0.024 | 0.53 | -0.068 | 0.48 |
| p-value | NS | NS | NS | NS |
| CD3+CD8+CD28+ |
| Correlation coefficient | -0.051 | 0.39 | 0.091 | 0.68 |
| p-value | NS | NS | NS | 0.03 |
No significant difference was observed in the ferritin level between the splenectomized and unsplenectomized patients (1487±995.85 vs. 1565±956 ng/dl). The number of CD3+CD8+CD27+ cells was inversely correlated with ferritin level (p < 0.01), while there was no correlation between this cell population and age, blood transfusion, and desferal infusion level. However, the percentage of these cells was significantly and inversely correlated with total blood transfusion and desferal infusion level (p = 0.03 and p = 0.02, respectively, data not shown in Table). The number of CD3+CD4+CD27+ lymphocytes was not correlated with age, blood transfusion, and ferritin level; however, the percentage of CD3+CD8+CD27+ cells was inversely correlated with the blood transfusion and desferal infusion level, as well (p = 0.04 and p = 0.02 respectively, the data not shown in Table).
The number of CD3+CD4+CD57+ lymphocytes was positively correlated with ferritin level [Table/Fig-6], while there was no correlation of this cell population with age, blood transfusion, and desferal infusion level. The number of CD3+CD8+CD57+ lymphocytes was also positively correlated with ferritin (p=0.003), but not with age, blood transfusion and desferal infusion level [Table/Fig-5,6].
a) A positive correlation was observed between the frequency of CD4+CD57+ T cells and ferritin level; b) A positive correlation was observed between the frequency of CD8+CD57+ T cells and ferritin level.
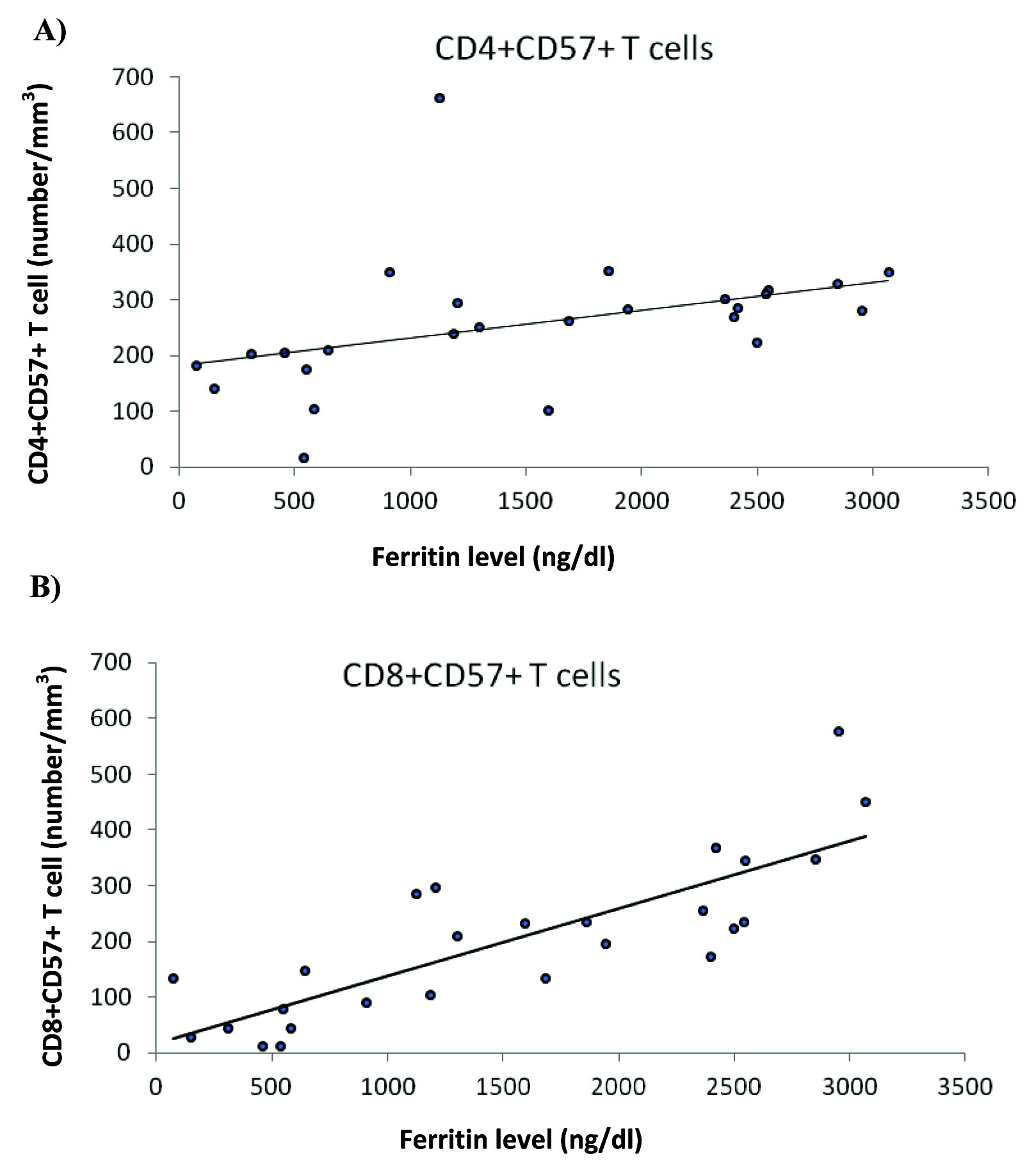
The number of CD3+CD8+CCR7+ cells was significantly correlated with ferritin level, while this cell population had no significant correlation with age, blood transfusion, and desferal infusion level. The number of CD3+CD4+CCR7+ cells was significantly correlated with none of the above variables, as well. CD28 expression in neither of the subgroups of CD4 and CD8 T cells was significantly correlated with the studied variables except for desferal infusion level with a positive correlation.
Discussion
Immunologic disorders in the patients with beta-thalassaemia major including non-specific immune response, lymphocyte subsets and changes in cytokines production have already been reported [7]. The mechanism of these disorders has not been clearly explained, but it could be related to iron overload, repeated blood transfusions, allogeneic stimulation and iron chelation therapy [14,16,17]. The significant increase in lymphocyte number is related to B cells number, which could be due to repeated blood transfusions. In our study CD4/CD8 ratio did not change significantly compared to the control group, which is consistent with the study conducted by by Al-Awadhi et al., [18]; however, in other studies an inversed ratio has been reported. Since the patients in our study were hepatitis B and C negative, normal CD4/CD8 ratio could be to some extent explained.
In investigating the correlation between the number of T lymphocytes and the patient’s age, ferritin level, blood transfusion, and desferal infusion level, no significant correlation was observed. Since CD3+ T cells have qualitative and quantitative changes in spelenectomized patients and 12 patients were splenectomized, the role of other factors could be moderated [19].
One of the findings in the present study is the moderate change in CD27 expression on T lymphocyte surface. Effector memory cells are CD27-, and in elderly people the decrease in CD27- expression on T lymphocytes surface has been noted [20]; in addition, the cells with a large amount of CD27 were not found among the virus-specific lymphocytes [21], which indicates these lymphocytes have lost the marker after stimulation. Both groups of effector memory cells, i.e. CD45RO+ and CD45RA+, are CD27- [22]. In B cells, the subgroup of IgD-CD27- lymphocytes increases as age advances [23]. In the study conducted by Albareda et al. a high proportion of CD45RA-CD27-CD28- was observed in the patients infected with tripanozome [24]. The HIV-infected patients with a higher dose of virus have a larger cell population of CD27-CCR7-CD45RO+ [25] In all these studies, chronic contact with antigen has negatively affected CD27 expression on lymphocytes surface and in our study expression rate of this molecule in the patients in both CD4 and CD8 populations was less than that in the control group, although the differences were not significant. The moderation of these changes could be related to the negativity of hepatitis B and C in these patients. Regarding limited number of the patients under our study, further research may be required.
No significant correlation was observed between the number of lymphocytes expressing CD27 and age, blood transfusion and desferal infusion level in CD4+ T cells, but the percentage of lymphocytes expressing CD27 was inversely correlated with blood transfusion and desferal infusion level. In CD8+ T cells, a negative correlation was observed with transfusion rate and ferritin level. Since contact with antigens increases as blood transfusion increases, these findings are not surprising. As transfusion of each blood unit could expose immune system to allogeneic antigens, it could be related to the moderate decrease in CD3+CD27+ lymphocytes. Increased ferritin level is correlated with iron overload in these patients and could affect immune system function adversely.
The number of CD3+CD4+CCR7+ lymphocytes in the control group was significantly higher than that of the patients. The expression of this marker is used to discriminate between naïve and central memory cells, and effectors memory cells [13]. The latter population is basically CCR7- [26]. The HIV-infected patients with high viral set point possessed significantly higher frequency of CCR7- T cells [25]. Less expression of this marker on CD4+ T cells in our patients could represent the decline in naïve and central memory versus effector memory (effector memory and terminally differentiated effector memory) T cells in these patients.
Another phenotypic index, CD57, significantly increased in the patients compared to the control group, was observed in both CD4+ and CD8+ T cells, but significant for CD4+ subgroup and partially significant for CD8+ T cells. This marker is a glycoprotein whose increase has been noted on the elderly’s T cells [27]. CD3+CD57+ cells are more sensitive to activation-induced cell apoptosis [9]. CD8+CD57+CD28- cells have a limited or no ability to proliferate; however, it has been challenged by some studies. They express that these cells are not completely scenescent and could proliferate while influenced by specific stimuli. Strioga et al., concluded that CD8+CD28-CD27- (CD8+CD57+CD27-) cells were closer to terminal differentiation and replicative senescence than CD8+CD57+CD27+ cells [9]. Increased proportion of CD57+ T cells in the peripheral blood of the patients with progressive gastric carcinoma has been associated with poor prognosis [28]. Expression of CD8+CD57+ T cells has been suggested as a diagnostic tool in the patients with an organomegally or organ infiltration [29]. Proportion of CD4+CD57+ T cells has been correlated with tumour progresssion and inversely correlated with IFNγ production in hepatocarcinoma patients [30]. The increased number of this population in our patients may be a feature of early immunosenescence. The correlation between the number of CD4+CD57+ T cells and ferritin level could explain the effect of iron overload on T lymphocyte subpopulations.
Conclusion
The results indicated that the patients with thalassaemia major who are hepatitis B, hepatitis C, and HIV negative had some changes in T lymphocytes. The moderate increase in subgroups of CD3+CD27-, CD3+CCR7-, and CD3+CD57+ cells could reflect nonspecific stimulation as well as the iron overload effect, potentially explained by negative correlation of blood transfusion and ferritin level with CD3+CD27- frequency as well as ferritin with CD3+CD57+ frequency. If the blood products containing younger red cells are used and consequently the need for blood transfusion and chelation therapy declines, early immunosenescence can be largely prevented in these patients. The new approaches to iron chelation like deferiprone should be further investigated. Since the present study was conducted on the patients over 10 years, study of younger patients is also needed to further investigate these changes and early immunosenescence. Moreover, investigating other immunosenescence-related alterations in these patients is suggested.