Persistent Mullerian Duct Syndrome with Ovarian Endometriosis-A Rare Case Report
Savitri Mallikarjun Nerune1, Surekha B. Hippargi2, Namrata B. Mestri3, Nikhil M. Mehrotra4
1 Assistant Professor, Department of Pathology, B.L.D.E.U’S Shri B.M.Patil Medical College Hospital & Research Centre, Vijayapur, Karnataka, India.
2 Professor, Department of Pathology, B.L.D.E.U’S Shri B.M. Patil Medical College Hospital & Research Centre, Vijayapur, Karnataka, India.
3 Postgraduate Student, Department of Pathology, B.L.D.E.U’S Shri B.M. Patil Medical College Hospital & Research Centre, Vijayapur, Karnataka, India.
4 Postgraduate Student, Department of Pathology, B.L.D.E.U’S Shri B.M. Patil Medical College Hospital & Research Centre, Vijayapur, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Savitri Mallikarjun Nerune, Assistant Professor, Department of Pathology, BLDEU’S Shri B.M. Patil Medical College, Vijayapur-586103, Karnataka, India.
E-mail: saviraj31j@gmail.com
Persistent Mullerian Duct Syndrome (PMDS) is a rare form of internal male pseudohermaphroditism, characterised by presence of Mullerian duct derivatives in a genotypic and phenotypic male. It is caused by absence of anti- Mullerian hormone or defective functioning of its receptors. We report a case of 19-year-old cryptorchid male with history of orchideopexy who was clinically and radiologically diagnosed as left sided chylocele. A definitive diagnosis of PMDS with ovarian endometriosis was made on histopathological examination which is important for genetic counselling and to reduce complications like infertility and neoplastic transformation. We report this case of PMDS with ovary showing evidence of endometriosis for its rarity.
Anti-Mullerian hormone, Male pseudohermaphrodite, Orchideopexy
Case Report
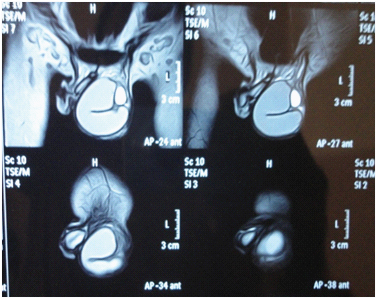
A 19-year-old boy presented with complaints of pain and swelling in the left scrotum since 15 days. There was a past history of left side cryptorchidism for which laparoscopic orchideopexy was done 7 years back. General physical examination revealed presence of bilateral gynaecomastia. On local examination swelling was noted in left inguinoscrotal region measuring approximately 12x8x6 cm. Right testis and external genitalia were normal. Hormonal profile showed elevated levels of Luteinizing Hormone (LH) and Follicle- Stimulating Hormone (FSH) while testosterone and prolactin levels were within normal limits [Table/Fig-1]. Ultrasound examination showed completely liquefied left testis and replacement with hypoechoic collection suggestive of testicular abscess/pyocele. On MRI of inguinoscrotal region, large multiloculated cystic collection noted in between layers of tunica vaginalis suggestive of high cholesterol / fat content- with features of chylocele [Table/Fig-2]. With the provisional diagnosis of pyocele, left inguinal exploration was planned and discussed with patient. Written consent was taken from the patient. Intraoperatively there was no pus but solid and cystic mass with attached tubular structure was found. As there was past history of orchideopexy, swelling was removed in toto and sent for histopathological examination.
Hormonal profile of the patient.
| Tests | Test Value | Reference Value |
|---|
| Testosterone | 4.47ng/Ml | Male: 3.0-10.6 |
| Prolactin | 24.2ng/Ml | Male: 3-25 |
| Female: 5-35 |
| Follicle Stimulating Harmone | 32iu/L | Male : 4-10 |
| Female: 10-20 |
| Luteinizing Harmone | 11.8miu/Ml | Male : 1.1-7.0 |
| Female : 2.0-2.5 |
MRI of left inguinoscrotal region (T1W1) show large multi loculated cystic collection in between layers of tunica vaginalis suggestive of high cholesterol / fat content- with features of chylocele.
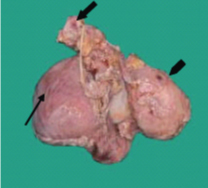
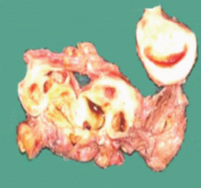
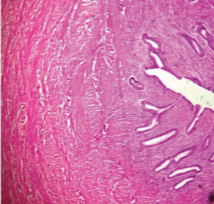
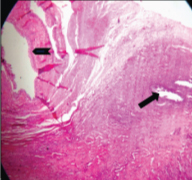
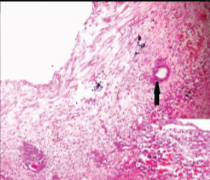
Gross examination of the specimen received showed a predominately cystic mass along with solid grey-white area and tubular structure. Cystic mass measured 10x8x2cm, a solid grey white nodule was 6x4x1.5cm in size with attached tubular structure measuring 10x2cm [Table/Fig-3a]. Cut section revealed multi-loculated structure filled with altered blood with adjacent solid area having slit like space and attached tubular structure [Table/Fig-3b]. Microscopic evaluation of the solid nodule showed endometrial glands embedded in compact stroma with adjoining normal myometrial tissue [Table/Fig-4a]. Multiple sections from the tubular structure showed fallopian tube mucosa composed of numerous delicate plical folds [Table/Fig-4b]. Sections from cystic mass showed ovarian tissue with cystic follicles, endometrial glands and stroma with haemosiderin laden macrophages in the ovarian stroma suggestive of ovarian endometriosis [Table/Fig-5a&b]. Neither malignancy nor testicular parenchyma was identified in spite of extensive grossing. So final diagnosis offered was PMDS with ovarian endometriosis. Genetic counselling was done and karyotyping was suggested but patient lost for follow up.
Gross photograph show cystic mass (thin arrow), tubular structure (think arrow) & solid nodule (arrow head).
Cut surface of specimen showing multiloculated cyst with altered blood and solid nodule with slit like space and attached tubular structure.
Photomicrograph of solid nodule showing endometrial glands in compact stroma and myometrium (H&E,100X).
Photomicrograph of tubular structure showing fallopian tube mucosa with plical folds (H&E, 400X).
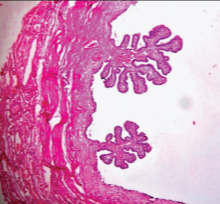
Photomicrograph of cystic mass showing cystic follicle of ovary (arrow head) with presence of endometrial stroma (arrow).
Photomicrograph of cystic mass show endometrial gland. Inset shows hemosiderin laden macrophages.
Discussion
PMDS is a rare form of internal male pseudohermaphroditism, characterised by presence of Mullerian duct derivatives (uterus, fallopian tube and upper part of vagina) in a genotypic (46 XY) and phenotypic male. It is thought to result from lack of anti- Mullerian hormone or defective functioning of anti-Mullerian hormone type II receptors [1]. This condition may occur sporadically or inherited as an X- linked, autosomal dominant or autosomal recessive pattern [2]. Nilson first described this entity in 1939 [3,4]. Approximately 150 cases of PMDS have been reported in literature mostly in adults and from Western, European and Middle Eastern settings [1,5]. PMDS with ovarian endometriosis is very rare. Even after careful search in literature, we could not find any case report of ovarian endometriosis in a patient of PMDS [1–7].
At 7 weeks of gestation, both Mullerian and Wolffian duct derivatives are present in human fetus. By the end of 7th gestational week testis differentiates in male fetus. Normal sex differentiation depends on testosterone, dihydrotestosterone and mullerian inhibiting factor (MIF) [6]. The human gene for MIF (also called as anti-Mullerian hormone) has been localised to short arm of chromosome 19p13.3 [3]. Sertoli cells secrete MIF which causes regression of Mullerian ducts in male fetus and testosterone secreted by leydig cells promotes the differentiation of Wolffian duct derivatives into epididymis, vas deferens and seminal vesicles [6]. Absence or deficiency of anti- Mullerian Hormone or defective functioning of its type II receptors causes persistence of Mullerian duct structures in male fetus [2]. Since secretion and action of testosterone is not affected, the Wolffian duct derivatives and external genitalia of the fetus develop as that of a normal male. Thus, PMDS is characterized by presence of Mullerian duct derivatives in genotypic (46XY) male and normal male external genitalia [2]. Present case showed presence of uterus and fallopian tube along with ovary showing evidence of endometriosis with normal male external genitalia.
Three anatomical variants of PMDS are encountered. The most common variant is male type in which one testis is found within the scrotum and the uterus and the ipsilateral fallopian tubes in the inguinal canal. In few cases the contralateral testis and tube are seen in hernia sac which is called as transverse testicular ectopia. The least common variant also called as female type shows bilateral cryptorchidism with the testes embedded in broad ligaments in an ovarian position with respect to the uterus, which is fixed in the pelvis [2,3,7].
Infertility is common in these patients. However, fertility is reported in few cases, although absolute proof of paternity was not established [4,5]. The indexed case is 19-year-old unmarried boy with three normal siblings.
Like other undescended testis, there is increased risk of malignant transformation in gonads of these patients. Germ cell tumours like embryonal carcinoma, seminoma, yolk sac tumour and teratoma have been reported in these patients, whereas tumours of mullerian duct derivatives are rare. The overall incidence of malignant transformation is around 15% [4–6]. In indexed case, neither testicular tissue nor any malignancy was identified in the multiple sections studied from the mass removed.
PMDS is diagnosed on the basis of clinical and radiological findings. Although imaging features of PMDS are classic, they are often missed [1]. In our patient ultrasound scan and MRI failed to identify the internal structures, but histopathological examination helped in diagnosis of PMDS. Ideally diagnosis of PMDS should be complemented by karyotyping, for which we lacked the requisite facility and patient willingness.
After confirmation of diagnosis, definitive treatment is aimed at preserving fertility and prevention of malignancies in undescended testis and mullerian duct remnants. Surgery consists of removal of the mullerian remnants with orchideopexy or orchidectomy. Because of possible chromosomal origin of this syndrome genetic counselling and long term follow-up should be offered to these patients [1–4].
Conclusion
To conclude, while dealing with the cryptorchid testis, the clinicians and pathologist should be aware of the entity of PMDS. This is important for diagnosis, treatment and genetic counselling of patient and to prevent complications like infertility and malignant transformation.
[1]. Odi TO, Abdur-Rahaman LO, Nasir AA, Persistent mullerian duct syndrome: A case report and review of literatureAfr J of Paediatric Surg 2010 7:191-93. [Google Scholar]
[2]. Sichani MM, Heidapour M, Dadkhah A, Rezavani M, Persistent Mullerian Duct Syndrome with an Irreducible Inguinal HerniaUrol J 2009 6:298-300. [Google Scholar]
[3]. Suresh N, Ganapathy H, Shekhar S, Prakashiny An interesting case of Persistent Mullerian Duct Syndrome –An incidental findingIOSR-JDMS 2014 13:44-46. [Google Scholar]
[4]. Prakash N, Khurana A, Narula B, Persistent Mullerian duct syndromeIndian Journal of Pathology and Microbiology 2009 52:546-48. [Google Scholar]
[5]. Kaore A, Kaore B, Persistent Mullerian Duct SyndromeNJIRM 2012 3:153-54. [Google Scholar]
[6]. Renu D, Rao BG, Ranganath K, Namitha Persistant mullerian duct syndromeIndian J Radiol Imaging 2010 20:72-74. [Google Scholar]
[7]. Patil V, Muktinaini S, Patil R, Verma A, Persistent Mullerain Duct Syndrome: a Case ReportIndian J Surg 2013 75:460-62. [Google Scholar]