Diallyl Disulphide (DADS) is present as essential oil in garlic, which is gathering interest due to its numerous biological activities [1,2]. Several epidemiological studies have suggested possible anticancer properties of DADS [3,4]. The anticancer property of garlic has been attributed to the presence of DADS and other organosulphur compounds [5]. DADS has shown to enhance gap-junctional intercellular communication by both direct and indirect mechanisms in rat liver cells thus counteracting the inhibition caused by the tumour promoters [6]. DADS also has shown therapeutic usage in methicillin resistant Staphylococcus aureus infections in BALB/cA mice [7]. Antifungal property against Allium white rot causing Sclerotium cepivorum has increased the yield of the crop [8]. DADS have shown to inhibit the growth of human breast cancer cells in culture [9]. Preventive effects of DADS in N-nitrosodiethylamine induced hepatocarcinogenesis have been reported [10]. DADS in combination with N-acetyl cysteine produced protection in acetaminophen hepatotoxicity with α-napthoflavone pretreated mice [11].
Electron beam is a concentrated, highly charged stream of electrons generated by accelerators capable of producing either pulsed or continuous beams [12]. It is a form of ionizing energy characterized by low penetration and high dosage rates. The samples during irradiation absorb energy from the electrons which causes various chemical and biological alterations [13]. This energy referred to as the ‘absorbed dose’ causes damage to the DNA of the reproductive cells of the organisms [14]. The major application of electron beam is in the field of sterilization of healthcare products and in the treatment of skin cancers.
In the present study, DADS was evaluated for its antioxidant, haematopoietic and membrane stabilizing properties in mice which have been irradiated with a sublethal dose of electron beam radiation.
Materials and Methods
Study Design
The study was conducted for a period of six months from December 2013 to June 2014 after procuring the essential requirements for the study. The study involved Swiss albino mice procured from the institution’s animal house. The effect of intervention was evaluated by comparing with changes in the corresponding drug control.
Ethical Approval
The study was ethically approved by the Institutional Animal Ethics Committee of the KS Hegde Medical Academy, Nitte University Ref. KSHEMA/IAEC/17/2013 dated 16.12.2013.
Chemicals
Commercially available diallyl disulphide was purchased from TCI chemicals pvt. Ltd., Japan. The other chemicals were purchased from CDH chemicals, Mumbai.
Invivo study
Male Swiss albino mice, 4-6 weeks old with 30±5g were selected for the study. They were maintained in an animal house with food and water ad-libitum. They were divided into 6 groups containing 6 mice in each group.
Drug Administration
The acute toxicity study conducted by [15] demonstrated 130mg/kg body weight of DADS was the median lethal dose. The 1st group served as control which was administered with distilled water. The radiation control (group 2) was given distilled water for 15 days and irradiated. the group 3 served as drug control and group 4, group 5 and group 6 were pre-treated with 13mg/kg, 26mg/kg and 52mg/kg body weight of DADS respectively were irradiated one hour after drug administration on the 15th day.
Irradiation of Mice
The irradiation work was carried out at the Microtron center, Mangalore University. On the 15th day, one hour after the administration of DADS, the mice were placed in well- ventilated perspex boxes with a dimension 3X5 cm. They were irradiated with a sub-lethal dose of 6Gy of electron beam radiation at a dose rate of 72Gy/min, with a source to target distance of 30cm [16].
Dissection of Mice
The mice were dissected after 24 hours of irradiation. The whole blood was collected by cardiac puncture for haematological estimations. The organs like liver, kidney, brain and spleen were dissected and weighed. The bone marrow was removed for cytological studies.
Haematological Studies
The haematological studies were done using Erma veterinary blood cell counter (PCE-210VET) using the whole blood collected in 2% EDTA.
The whole blood was collected in 2% EDTA tubes and processed for haematological studies within 3 hours of collection. The serum/ plasma were separated and stored in Panasonic (MDF-U334-PE) biomedical freezer at-300C until further processing.
The spleen removed from the mice was weighed and the splenic index was calculated using the formula:
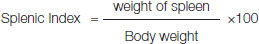
Antioxidant Studies
Suitable spectrophotometric methods were used and the measurements recorded in Systronics PC based double beam UV spectrophotometer 2202.
Total Antioxidant Capacity [
19]
In this assay, 100μL of sample was treated with 100 μL of tri-chloroacetic acid. The mixture was allowed to stand for 5 minutes and centrifuged at 3000 rpm and the supernatant was separated. 100 μL of supernatant was taken and 1ml of molybdic acid reagent was added. The mixture was kept in a boiling waterbath for 90 minutes. The absorbance was read at 695nm. The molybdic acid reagent contained 0.6M sulphuric acid, 28mM sodium dihydrogen ortho-phosphate and 4mM ammonium heptamolybdate.
Superoxide Dismutase [
20]
The superoxide dismutase activity was determined by treating 0.1mL of sample with a mixture of methionine, riboflavin and nitro blue tetrazolium chloride. This mixture was allowed to stand 10 minutes under fluorescent light. The blue-green coloured solution was read at 560nm. The activity of superoxide dismutase was expressed in units/mg of haemoglobin for haemolysates.
Glutathione Peroxidase [
21]
The glutathione peroxidase activity was measured by treating 0.25mL of sample with 0.2mL of 400mM phosphate buffer, 0.05mL of 10mM sodium azide, 0.1mL of 4mM reduced glutathione and 2.5mM hydrogen peroxide. The reaction mixture was incubated at 370C for 30 minutes and centrifuged at 3000rpm for 10 minutes. 1ml of the supernatant was treated with 0.25mL of 10% tri-chloroacetic acid, 1.5 ml of 0.3M phosphate buffer, 1mL distilled water and 0.25mL of 5, 5’- dithio bis (2- nitrobenzoic acid) (Ellman’s reagent). The absorbance was measured at 412 nm and the activity expressed in units/mg of protein for homogenates and units/mg of haemoglobin for haemolysates.
Glutathione S-Transferase [
22]
The glutathione S- transferase activity was measured by treating 0.1mL of sample with 0.1mL of 1mM reduced glutathione, 2.6mL of 0.1M phosphate buffer (pH-6.5) and 0.1 mL of 1-chloro -2, 4 –dinitrobenzene. This kinetic measurement was taken at 240 nm with 15 seconds delay for 3 minutes. The activity was expressed in units/mg of protein for homogenates and units/mg of haemoglobin for haemolysates.
The catalase activity was determined by treating 10 μL of sample with 3ml of 60mM hydrogen peroxide. The kinetic measurement was taken at 240nm with 30 second delay for 2 minutes. The activity was expressed in units/mg of protein for homogenates and units/mg of haemoglobin for haemolysates.
Lipid Peroxidation and Membrane Stabilization
Formation of Malondialdehyde [24]
The formation of malondialdehyde was estimated by diluting 0.1mL of sample with 0.4mL of distilled water and 1mL of trichloroacetic acid – thiobarbituric acid reagent (TCA-TBA reagent). The reaction mixture was kept in a boiling waterbath for 15minutes. The endpoint was measured at 535nm and calculated using malondialdehyde standard curve.
Reduced Glutathione [25]
The reduced glutathione was estimated by treating 0.1mL of sample with 1.5mL of precipitating solution containing meta-phosphoric acid sticks and sodium chloride. The mixture is allowed to stand for 10 minutes and centrifuged. 0.5mL of this supernatant was treated with 2mL of 0.3M phosphate solution and 0.25mL of 5, 5’- dithio bis (2- nitrobenzoic acid). The absorbance was read at 412nm within 10 minutes and calculated using a GSH standard curve.
Evaluation of Cytotoxicity [26]
The cytotoxic effect of the drug was evaluated in bone marrow cells. The bone marrow was flushed with 5% bovine serum albumin (BSA) and smeared on a clean glass slide. The smear was then fixed in methanol, stained with May-Grunwald stain and Giemsa stain. The slide was then dried and the number of polychromatic erythrocytes and norchmochromic erythrocytes with or without micronucleus were counted and expressed in percentage.
Statistical Analysis
All the values are expressed as mean ± standard deviation. Analysis of data and comparison was done using one-way ANOVA with Tukey’s multiple comparison test using PRISM 3.0 software. The p-value less than 0.05 were considered significant.
Results
The haematological changes in control, radiation control and DADS treatment prior irradiation is shown in [Table/Fig-1]. The antioxidant parameters in control, radiation control and drug + radiated groups with 3 different doses of diallyl disulphide are depicted in [Table/Fig-2]. The MDA and reduced glutathione levels in control, radiated and DADS + radiated groups with 3 different doses of diallyl disulphide is shown in [Table/Fig-3a&b]. [Table/Fig-4] shows the splenic index in control (C), radiated (R) and drug + radiated groups with 3 different doses of diallyl disulphide (13mg, 26mg and 52mg/kg body weight: D1, D2, D3). [Table/Fig-5] shows the percentage of micronucleus formation in control (C), radiated (R) and drug + radiated groups with 3 different doses of diallyl disulphide (13mg, 26mg and 52mg/kg body weight: D1, D2, D3). [Table/Fig-6] is showing the IL-6 levels in control (C), radiated (R) and drug + radiated groups with 3 different doses of diallyl disulphide (13mg, 26mg and 52mg/kg body weight: D1, D2, D3).
Showing the blood cell count obtained in Control, Radiated and Drug + Radiated groups with 3 different doses of Diallyl Disulphide (13mg, 26mg and 52mg/kg body weight).
| Groups | Total RBC Count(Cells/μL) | Haemoglobin (g%) | % Haematocrit (PCV) | Total WBC Count(Cells/ μL) | Platelet Count/ μL |
|---|
| Control | 7157500 ± 981765.5 | 12.2 ± 2 | 30 ± 5 | 4126 ± 200 | 393000 ± 70934 |
| Radiation control | 4333333 ± 971716.7** | 6.1 ± 1.15** | 21.83 ± 5.53*** | 1466.66 ± 602.77*** | 219000 ± 67882.25** |
| Group 1 DADS (13mg/kg) | 5177500 ± 621040.8* | 7.95 ± 1.28** | 27.525 ± 3.09** | 1050 ± 450.925*** | 283500 ± 16901.68* |
| Group 2 DADS (26mg/kg) | 6885000 ± 745587* | 10.475 ± 1.05* | 36.15 ± 3.16*** | 4733.33 ± 1200** | 327666.7 ± 46736.85 |
| Group 3 DADS (52 mg/kg) | 6496667 ± 680392* | 9.4 ± 1.25* | 33.1 ± 3.02** | 1466.67 ± 351.1885*** | 368500 ± 139300* |
The values are expressed as Mean ± SD; * p-value <0.05, **p-value <0.01, ***p-value <0.001
Showing the antioxidant parameters in control, radiation control and drug + radiated groups with 3 different doses of diallyl disulphide.
| Groups | Superoxide dismutasemU/mg Hb | Total antioxidant capacityμM/mL |
|---|
| Control | 6.84±0.9 | 0.062±0.02 |
| Radiation Control | 7.55±0.3 | 0.00667±0.001*** |
| Group 1 DADS (13mg/kg) | 5.70±0.2 | 0.0002±0.0001*** |
| Group 2 DADS (26mg/kg) | 4.10±0.2 | 0.0705±0.055 |
| Group 3 DADS (52mg/kg) | 5.11±0.8 | 0.0001±0.0002*** |
The values are expressed as Mean ± SD; * p-value <0.05, **p-value <0.01, ***p-value <0.001
The results of catalase, GPx and GST are not shown as their results are not significant. (p>0.05)
Showing the Biochemical Analyses done in Control (C), Radiation Control (RC), and DADS + radiated groups with 3 different doses of DADS (13mg, 26mg and 52mg/kg body weight: D1, D2, D3).
| Parameter | Control | Radiation control | Group 1 DADS (13mg/kg) | Group 2 DADS (26mg/kg) | Group 3 DADS (52mg/kg) |
|---|
| Urea (mg/dl) | 5.4 ±1.2 | 102±10.2** | 88±8.8 | 61±6.1 | 113±11.3 |
| Creatine (mg/dl) | 0.3±0.2 | 0.6±0.3 | 0.6±0.4 | 0.5±0.4 | 0.6±0.4 |
| Bilirubin (mg/dl) | 0.8±0.2 | 0.9±0.3 | 0.7±0.4 | 0.8±0.4 | 0.9±0.3 |
| Total Protein (g/dl) | 9±1.2 | 8±1.1 | 8±1.2 | 7.5±1.5 | 8.8±0.9 |
| Albumin (g/dl) | 3.8±1.2 | 3.6±1.4 | 3.4±1.4 | 3.5±1.2 | 3.7±0.9 |
| SGOT (U/L) | 160±22 | 670±78.5 | 730±78 | 510±85.4 | 1230±201.3 |
| SGPT (U/L) | 44±4.4 | 330±33** | 250±25* | 170±17* | 380±38** |
| ALP (U/L) | 50±26.3 | 80±35.4 | 110±45 | 170±28.5 | 170±35.6 |
The values are expressed as Mean ±SD; *p-value <0.05, **p-value <0.01, ***p-value <0.001
Showing the MDA and reduced glutathione levels in control, radiated and DADS + radiated groups with 3 different doses of diallyl disulphide.
| Groups | MDA(μM/L) | Red. GSH(μg/mL) |
|---|
| Control | 0.63±0.44 | 1.9±0.69 |
| Radiation Control | 1.64±0.83 | 1.42±0.12 |
| Group 1 DADS (13mg/kg) | 0.658±0.25 | 1.39±0.45 |
| Group 2 DADS (26mg/kg) | 0.822±0.49 | 3.14±1.10 |
| Group 3 DADS (52mg/kg) | 1.05±0.24 | 3.64±1.17 |
The values are expressed as Mean ±SD; *p-value <0.05, **p-value <0.01, ***p-value <0.001
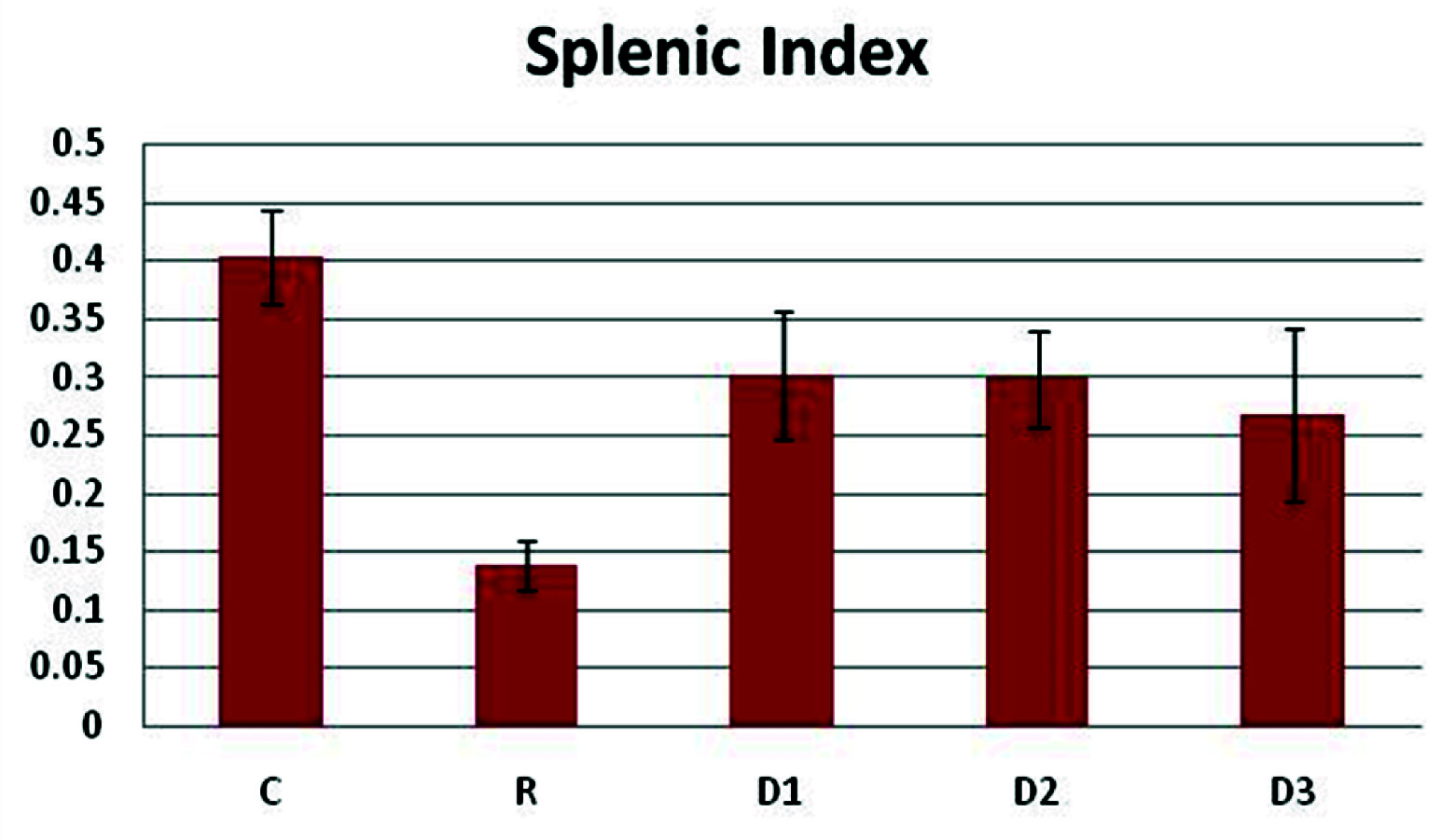
Showing the splenic Index in Control (C), Radiated (R) and drug + radiated groups with 3 different doses of diallyl disulphide (13mg, 26mg and 52mg/kg body weight: D1, D2, D3).
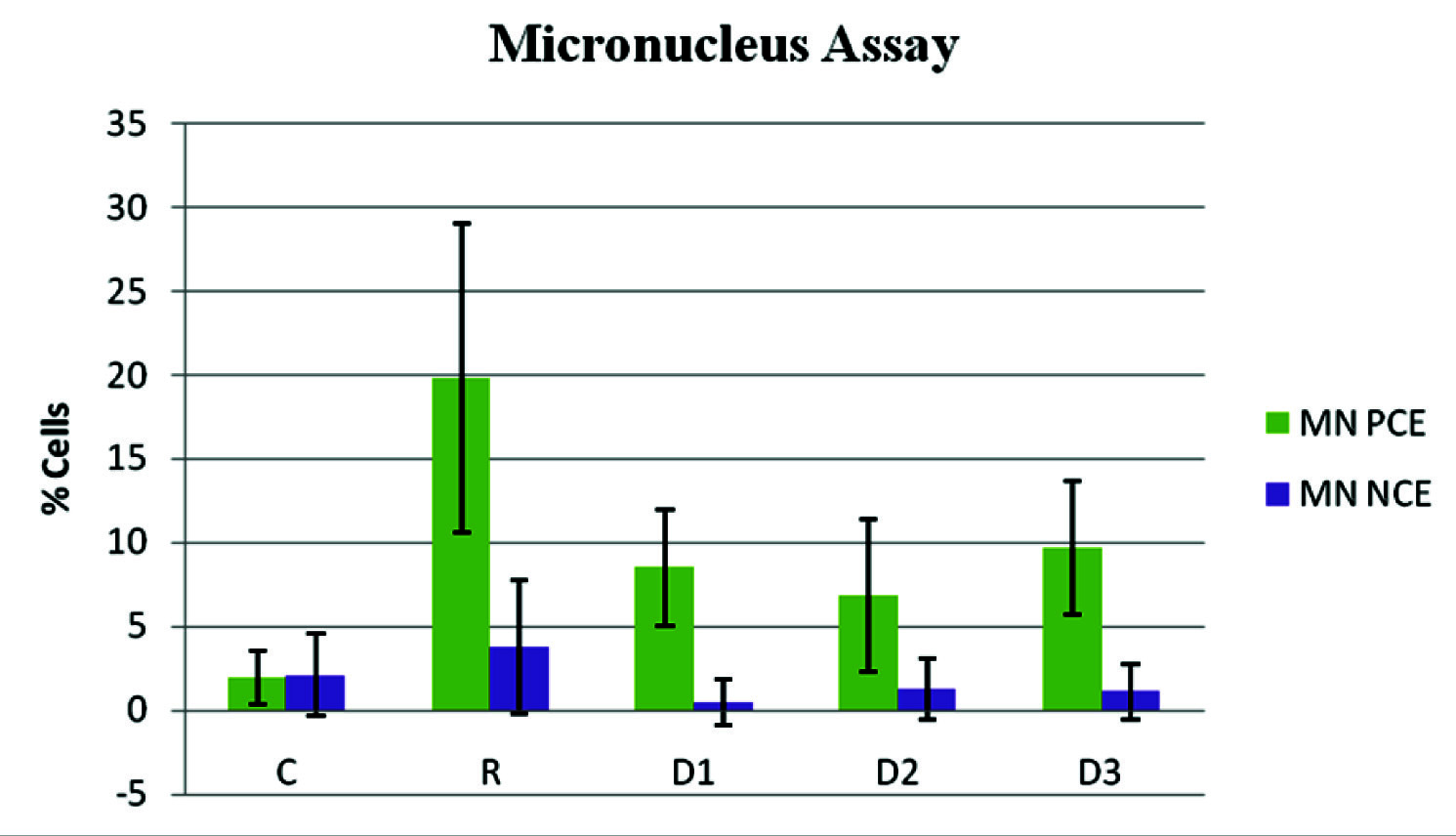
Showing the percentage of micronulceus formation in control (C), radiated (R) and drug + radiated groups with 3 different doses of diallyl disulphide (13mg, 26mg and 52mg/kg body weight: D1, D2, D3).
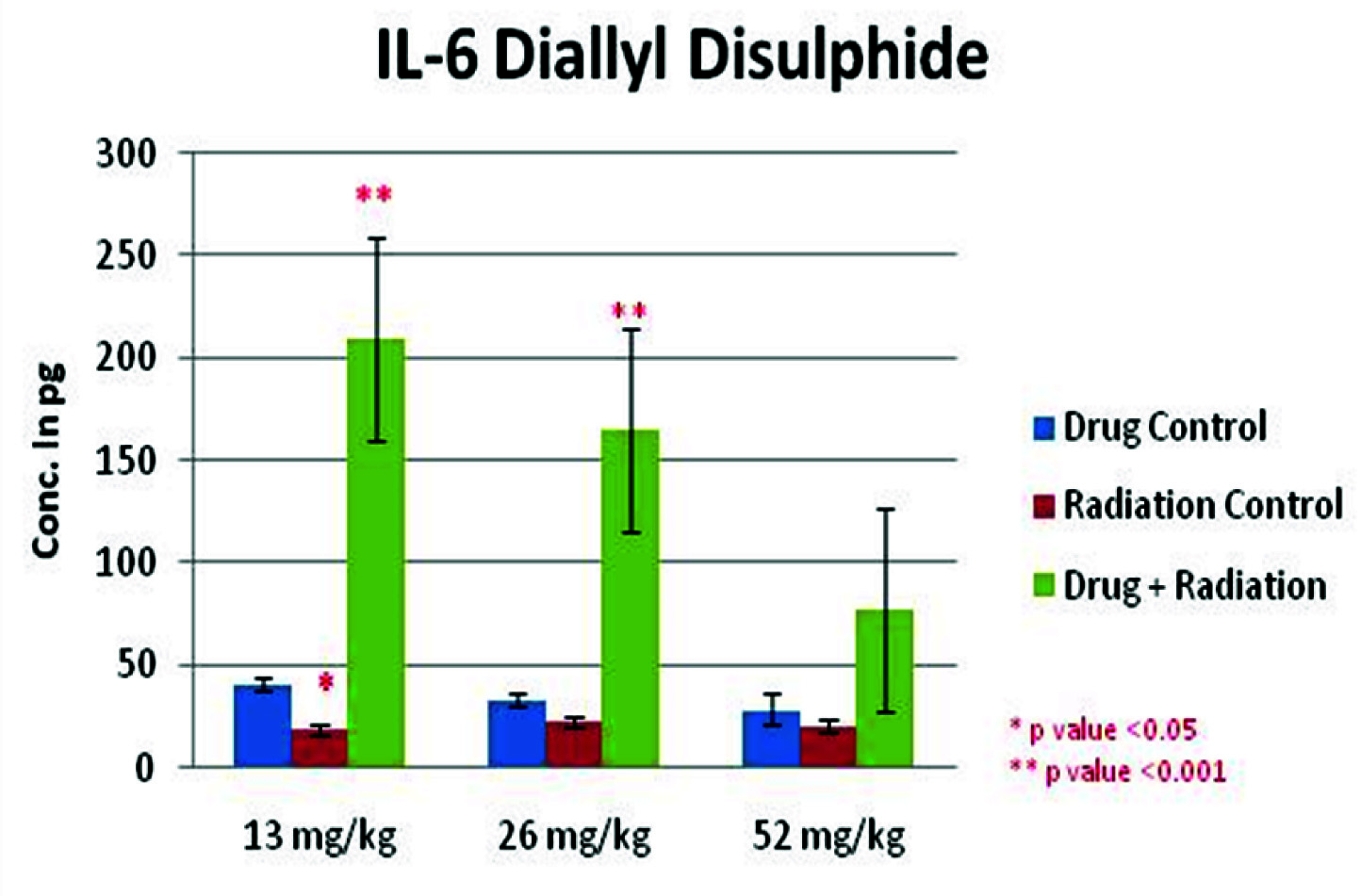
Showing the IL-6 levels in control, radiated and DADS + radiated groups with 3 different doses of DADS (13mg, 26mg and 52mg/kg body weights).
Discussion
The deleterious effects of radiation include formation of free radicals and development of oxidative stress. Sulphydryl compounds like amifostine [27] cysteine, cysteamine, amino ethyl thiourea, glutathione [28] have been shown to reduce the radiation induced tissue damage by scavenging the free radicals or by repairing the radioactivated polymers or activating the repair enzymes [29]. Diallyl sulphide (DAS), diallyl disulphide (DADS), allyl methyl sulphide (AMS), allyl isothio cyanate (AITC) and phenyl isothio cyanate (PITC) which is naturally occurring organosulphur compounds were shown to enhance the glutathione content contributing to their radioprotective effect [4]. Protective effects were only seen in the presence of these compounds in the system prior irradiation [30]. Hence sulphydryl compounds only have prophylactic effect and no therapeutic effect. Intra cellular non-protein sulphydryl groups are mainly involved in enhancing the membrane stability and antioxidant capacity.
Acute toxicity of DADS was studied in female Swiss albino mice. The results reveal that at 150mg/kg body weight, all the animals died within 24 hours after oral administration. We obtained a median lethal dose of 75mg/kg body weight. Previous study by [15] recorded the median lethal dose at 135mg/kg body weight in females and 150mg/kg body weight in males. The results of current study reveal diallyl sulphide to be highly toxic at those doses.
The results of haematology indicate a significant increase in the haemoglobin levels, enhancement in the number of red blood cells, haematocrit, white blood cells and platelets in DADS pretreatment prior radiation groups compared to radiation control group (p<0.01). The haematological enhancement has been at a concentration of 26mg/kg body weight of DADS. DADS has shown a notable antihaemolytic property invitro [31]. A reduction in the splenic index of the DADS pretreatment prior radiation groups compared to the radiation control indicates a possible decrease in the haematopoietic stress on the spleen. An increase in the interleukin-6 levels was seen in the DADS pretreatment prior radiation groups when compared to the radiation control group. Interleukin-6 has been shown to play a key role in the induction of immune response and stimulation of haematopoiesis [32]. There is enhancement in the reduced glutathione levels (p<0.01) in the DADS pretreatment prior radiation groups compared to the radiation control group. A notable reduction was seen in the malondialdehyde levels in DADS pretreatment prior radiation groups compared to the radiation control, in the red cell lysates. These results indicate the haematopoietic and membrane stabilization potential of DADS. At 26mg/kg body weight, DADS has shown enhancement in the total antioxidant capacity compared to the radiation control. Decrease in the cytogenetic damages can be seen as a reduction in the micronucleus formation in the polychromatic and normochromatic erythrocytes. Radiation has clastogenic effects on the bone marrow cells [33] and this effect has been reduced by around 10% in the DADS pretreatment prior radiation groups when compared to the radiation control groups. The probable explanation may be that DADS has increased the intracellular non-protein sulphydryl content and has been able to minimize the oxidative damage. Also a dose dependant reduction was observed in the interleukin-6 levels in the different doses of DADS with highest levels of IL-6 at 13mg/kg and reduced markedly at 52mg/kg body weight. The exact reason for this is unknown but it is possible that at a high dose there is increased level of toxicity as are other sulphydryl compounds at their highest doses. DADS has been shown to have hepatoprotective effects in previous studies [34] but no significant change in the liver and kidney function were seen in the DADS pretreatment prior radiation groups compared to the radiation control group. No significant changes were observed in the enzymatic antioxidants like superoxide dismutase, catalase, glutathione peroxidase, glutathione-S-transferase which play an important role in the free radical scavenging.
A limited literature is available to discuss about post-radiation studies supporting the therapeutic aspects of radioprotective drugs. DADS also did not show any significant protective effect when compared to the radiation control group. As the radiation dose was sublethal in this study, it is obvious that the repair mechanism has already taken place and has affected the result and the negative result could also be that DADS could have enhanced apoptosis in the cells which have already been damaged due to radiation induced oxidative stress. Also a combination of pre and post study group of DADS prior radiation did not yield any significant result probably because of the same reasons. But a pre and post treatment combination of a drug could be helpful in antioxidant replenishment in radiotherapy.
Conclusion
From this study we can conclude that DADS provided protection against radiation induced damages in mice by enhancing the heamatopoietic, antioxidant and membrane stabilization. Further the study can be extended to study the dose dependant changes and effects in cancer models to develop DADS as radioprotective agent.
The values are expressed as Mean ± SD; * p-value <0.05, **p-value <0.01, ***p-value <0.001
The values are expressed as Mean ± SD; * p-value <0.05, **p-value <0.01, ***p-value <0.001
The results of catalase, GPx and GST are not shown as their results are not significant. (p>0.05)
The values are expressed as Mean ±SD; *p-value <0.05, **p-value <0.01, ***p-value <0.001
The values are expressed as Mean ±SD; *p-value <0.05, **p-value <0.01, ***p-value <0.001