Widespread use of fluoride has proven to be a major factor in reducing prevalence and severity of dental caries. Although decline is a major achievement, still considerable burden of disease is seen in all age groups [1].
Dental restorative materials serve as one of the fluoride delivery method which confers resistance not only to the tooth restored but also to the adjacent tooth. Among the various materials available, Glass ionomer cements (GIC) have gained preference owing to its unique properties of chemical adhesion, acceptable aesthetics, biocompatibility and fluoride release and recharging ability [2]. Incipient carious lesions close to GIC have been shown to remineralise or even hypermineralise in comparison to amalgam and composite with continuing demineralisation [1].
However, the clinical use of GIC still remains limited because of its relative inferior mechanical properties and sensitivity to initial desiccation and moisture [3]. To overcome these shortcomings various modifications have been introduced like giomer, compomer and resin-modified GIC. However research continued in the search of biocompatible and potentially adhering additive which will not only improve the shortcomings but will also retain the inherent unique anticariogenic property of GIC which is fluoride release and rechargability. Recently, Hydroxyapatite (HA) whiskers have been introduced as biomimetic strengthening additive which has been proven to improve its mechanical properties. Also, its addition did not affect significantly its fluoride releasing property showing similar release profile as GIC [4,5].
The ability to take up and re-release ions from exogenous sources is an important asset to GIC, which allow them to maintain incessant level of fluoride, thus serving as fluoride reservoirs [6]. Fluoride can be replenished with topical application of fluoride in gel, rinse or toothpaste. On daily basis, the most common source of fluorides is fluoridated dentifrices [7]. However, this unique refluoridation property of GIC is yet to be evaluated when HA whiskers are added. Keeping these established facts in mind, an invitro study was designed to evaluate the effect of addition of hydroxyapatite whiskers on recharging ability of GIC. As GIC is most widely used in paediatric practice hence low fluoride dentrifice (500ppm) was selected to evaluate the effect.
Hence the aim of the study was to evaluate the fluoride rechargibility of HA modified GIC with low fluoride dentrifice in comparison with conventional GIC.
Materials and Methods
An invitro study was designed using conventional and hydroxyapatite incorporated GIC. After obtaining ethical clearance from our institution (Institute of Dental Science and Hospital, Bhubaneshwar) the study was carried out in the year 2014. Sample size was decided taken into consideration other fluoride invitro studies available in literature [5,8]. Teflon mould (5mm diameter x 2mm height) and customised jig was designed for the preparation of samples. Forty (40) specimens (twenty of each material) were made by placing the restorative material into a Teflon mould slot supported by a glass slide. Vaseline coated mylar strip was placed, over which second glass slide was positioned over the Teflon mould. Screws in the vertical arms of the jig were gradually tightened to apply uniform gentle pressure so that the excess material flushes out. After the final setting as per manufacturer’s instructions the samples were demoulded and evaluated.
All specimens were then suspended individually in 25 ml of deionised water stored at 37oC. After 24 hours containers were shaken properly and samples were removed, dried and transferred to container containing 25ml of deionised water, for 21 days. After 7 days, test specimens were subjected to daily fluoride exposure protocol. At the predetermined time, each specimen was taken out and brushed with powered toothbrush using 0.38% w/w sodium monofluorophosphate dentifrice (500 ppm) for 1min twice daily for 21 days.
Buffering of media solutions was done by adding 5ml of total ionic strength adjusting buffer (TISAB II). Fluoride release of sample was measured every 24 hours after fluoride treatment for 7 days and weekly from the 7th day to 21st day using Sension4 pH / ion selective electrode.
Statistical Analysis
The data thus obtained from the experimental procedure was tabulated and statistically analysed using repeated measures ANOVA. The p-value of <0.05 was considered to be significant.
Results
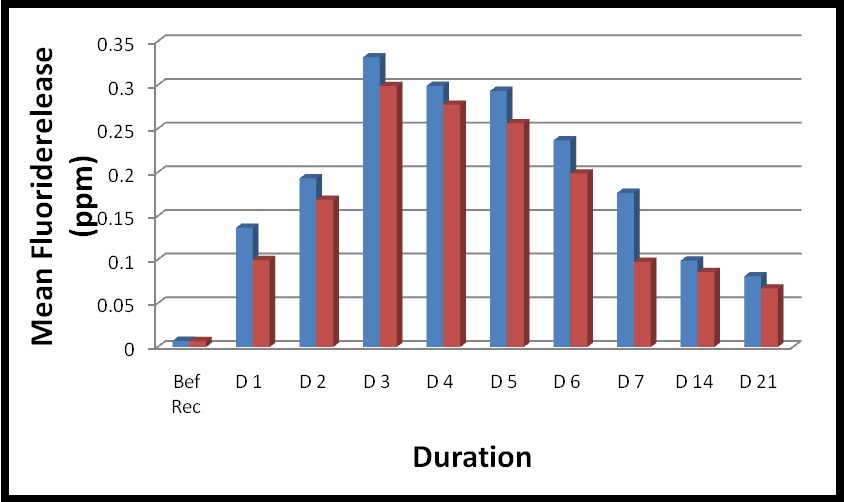
[Table/Fig-1] shows mean (± S.D) of fluoride release following recharging daily from day 1 to day 7 and weekly thereafter till 21 days. Highly significant increase (p<0.01) in amount of fluoride release was observed from both materials immediately after recharge. Both the materials showed identical pattern of release of fluoride during the course of the study. Fluoride release following recharge tended to be related to topical fluoride application.
Mean (±S.D) fluoride release (ppm) for each material following recharging from day1 to day 21.
| Before recharge | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 14 | Day 21 |
|---|
| HA-GIC | 0.0070±0.09 | 0.1366±0.09 | 0.1933±0.08 | 0.3319±0.42 | 0.2992±0.06 | 0.2934±0.07 | 0.2368±0.07 | 0.1766±0.08 | 0.099±0.08 | 0.0811±0.04 |
| GIC | 0.0068±0.08 | 0.0996±0.08 | 0.1607±0.08 | 0.2989±0.09 | 0.2776±0.17 | 0.2564±0.16 | 0.1988±0.25 | 0.0975±0.24 | 0.0859±0.06 | 0.0671±0.05 |
| p-value | | 0.009* | 0.000** | 0.004* | 0.036* | 0.144 | 0.003* | 0.008* | 0.172 | 0.000** |
Repeated measures ANOVA: p-value. ** 0.000-0.010: highly significant, *0.010-0.050: significant, >0.05: non-significant
For both the materials GIC and HA-GIC, greatest increase in fluoride release was observed from Day 1 to Day 3. By Day 1, fluoride release had significantly increased as compared to baseline value for GIC and HA-GIC (p=0.014 and p=0.009 respectively).
After Day 7, significant decrease in fluoride release was observed in HA-GIC (p=0.004) and in GIC (p=0.003) which continued to decrease attaining plateau reaching till Day 21. However, the fluoride release from both the material on Day 21 was significantly higher than baseline value pre-fluoride treatment.
For both the materials, greatest increase in fluoride release was seen from day 1 to day 7, following that there was insignificant difference from day 14 to day 21.
Repeated measures ANOVA showed statistically insignificant difference between two restorative materials demonstrating similar patterns of fluoride release over the time interval [Table/Fig-2].
Comparison of fluoride release (ppm) following recharging between HA-GIC and GIC from day 1 to day 21.
Discussion
Changing perception in era of minimal intervention dentistry along with raising consciousness of people has shifted the approach towards dental caries from merely techniques to biomedical approach. Thus, modern approach aims at controlling dental caries requires dental materials possessing both restorative and prophylactic properties. With ever increasing demands now it is more desirable to gain better outcome with less invasive techniques.
Wilson and Kent in 1972 introduced a unique cement to dentistry, glass ionomer cement (GIC) [3]. Especially in Paediatric dentistry, GICs have been used predominantly because of properties such as handling, fluoride release and recharge, Conferring its anticariogenic potential in addition to its biocompatibility and thermal expansion similar to teeth. GIC has been criticised because of its shortcomings like initial desiccation and poor mechanical properties which has limited its use to non-stress bearing areas but yet till date is the most popularly used among all clinicians. However, unceasing improvements aiming to improvise its shortcomings have led to its metamorphosed form like giomer, compomer, resin-modified GIC and many more [9].
GIC has an edge over other restorative materials because of its ability to recharge fluoride from external sources, thus maintaining sustained level of fluoride acting as fluoride reservoir and thus reducing demineralisation.
The urge for improvising its mechanical properties by addition of biologically compatible additives has led to the search of hydroxyapatite whiskers as strengthening material. Studies have shown that addition of sufficient amount of whiskers increased the flexural strength and improved the microstructure of GIC. It has also been documented that the fluoride release is not significantly affected by addition of hydroxyapatite whiskers, thus maintaining its inherent properties [5,10].
Studies have shown that Resin-modified GIC exhibited fluoride release and uptake capacity over the longer period but the mechanical properties was not greatly improvised in comparison of GIC [11]. In the dearth of literature this invitro study was designed to evaluate the recharging ability of Hydroxyapatite incorporated GIC in comparison with conventional GIC with fluoride recharging regimen with low fluoridated dentifrice. Hydroxyapatite whiskers have been proposed to enhance the mechanical properties of GIC without significant effect on its anticariogenic property. Different weight percentages (8%, 19 % and 25%) have been evaluated by various researchers. It was proven that maximum increase in flexural strength was with volumetric addition of 8% HA [5,10]. Thus in our study 8% hydroxyapatite whiskers was added volumetrically to the powder of conventional GIC.
Samples were prepared by placing the restorative material into Teflon mould supported by glass slides using customised mounting jig, designed to apply gentle uniform pressure extruding excess material [5,8].
Deionised water was used as the storage medium as it is easily obtainable and more fluoride is released as compared to artificial saliva. As deionised water does not contain any traces of fluoride, it can be considered as more absolute means to assess fluoride as releasing from the restorative material [11].
Various means of topical applications of fluoride have been described by many authors. The protocol followed in the present study is based on the rationale that most people expose their teeth to topical fluoride by use of fluoridated dentifrices or use of mouth rinses containing fluoride. Freedman had concluded that home care exposure of fluoride provide sufficient measurable fluoride uptake and re-release. However the protocol followed in present study differs from the one used by Freedman et al., in relation to the concentration of fluoride used for recharging [8,11]. In the present study fluoridated dentifrices containing 500ppm of fluoride was used keeping in mind the development of low fluoride paediatric dentifrices and to ascertain if recharging would occur at a lower level than adult fluoridated dentifrices (1000-1500ppm).
Among the various available methods for fluoride estimation, fluoride ion selective electrode was used in the study to evaluate fluoride concentration. Fluoride level was recorded for both the restorative material before recharging as baseline value and subsequent recordings were taken daily for 7 days and after that weekly once till 21 days. The difference between the values from the baseline value and between the days indicated the recharge potential of the particular restorative material. In agreement with previous researches, the present results also proved that exposure to fluoridated dentifrices allowed the material to take up fluoride [12]. Numerous studies have been performed in past in order to determine the concentration of fluoride release from GIC but precise mechanism of release is not yet fully elucidated. Fluoride release from GIC following recharge is mainly thought to be occurring in two ways. Short term release of fluoride because of leaching of loosely bound fluoride from surface adsorbed layer and long term release which is mainly diffusion controlled phenomenon governed by concentration gradient [13].
Among the various modifications of GIC available, only resin modified GIC showed potential to recharge but initial burst of fluoride release was not observed. Giomer and Compomer though have improved mechanical properties but fluoride release and recharging potential is deleteriously affected. It is also been established that materials with higher initial fluoride release have high recharge capacity [12,14].
Hydroxyapatite incorporated GIC tested in the present study was found to be capable of fluoride uptake as well as subsequent release as seen in conventional GIC. Both HA-GIC and conventional GIC showed initial burst of fluoride concentration following recharge and continued to increase till 3rd day. After which it started declining till 7th day finally reaching a plateau extending upto 21st day.
Significant increase in mean (±S.D) fluoride level in HA-GIC was recorded to 0.1366±0.09 ppm and in conventional GIC 0.0996±0.08ppm following recharge as compared to baseline value, however difference between the testing groups was found to be insignificant. The pattern of fluoride recharge of HA-GIC was found similar to conventional GIC, after sharp rise followed by the rapid decline and then gradual prolonged sustained release [15]. The pattern of recharge and re-release obtained in the present study was in agreement with various other authors [11,14]. However initial outburst was not observed in the other modifications of GIC including RM-GIC and Giomer which showed potential to recharge [12,16].
In the present study, the mean (±S.D) fluoride on Day 21 in group HA-GIC was 0.0811±0.04 ppm and conventional GIC was 0.0671±0.05 ppm, which were considerably higher than values before recharge. This was in contrary to findings of Freedman et al., who stated that only surface adsorption of fluoride was mainly responsible for the initial increase, culminating in return to values lower or similar to before recharge values [2,13].
Limitation
It is important to note that there were a few shortcomings of the present study design like; quantification of fluoride was based on differences in the concentration of immersion media before and after recharge, while actual fluoride release from matrix needs further elaboration. Definitive conclusion cannot be drawn and further in vivo study is needed to evaluate the fluoride release after fluoride recharging in dynamic condition of oral cavity.
Conclusion
With the present modification of Glass ionomer with hydroxyapatite it is clearly proven that it increases the strength, allows for high degree fluoride release and maintain the unique ability of recharge and re-release thus aiding to serve as slow continuous fluoride release device. These can specifically be a great boon to paediatric practice and caries prone people.
Repeated measures ANOVA: p-value. ** 0.000-0.010: highly significant, *0.010-0.050: significant, >0.05: non-significant