Correlation of Oxidative Damage with Pro-Inflammatory Markers (IL-6, TNF-α) in Meningocele
Bedabrata Mukhopadhyay1, Roshni Gavel2, Ajay N. Gongopadhyay3, Pooja Vashistha4, Anjali Rani5, Surendra Pratap Mishra6
1 Department of Biochemistry, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India.
2 PG Resident, Department of Biochemistry, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India.
3 Senior Professor and Ex- HOD, Department of Paediatric Surgery, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India.
4 PG Resident, Department of Biochemistry, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India.
5 Associate Professor, Department of Obstetrics and Gynecology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India.
6 Associate Professor, Department of Biochemistry, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Surendra Pratap Mishra, Associate Professor, Department of Biochemistry, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh-221005, India.
E-mail: drsurendram2@gmail.com
Introduction
Oxidative damage induces alteration in the status of pro-inflammatory markers like IL-6 and TNF-α in meningocele. The study was performed with estimation of the levels of MDA (Malonyldialdehyde), SOD (Superoxide dismutase) taken as oxidative damage markers and IL-6 (interleukin 6) and TNF-α (Tumour necrosis factor alpha) taken as inflammatory markers, in the serum of meningocele patients and age, sex matched normal neonates. Correlation among the different serum levels of MDA, SOD, IL-6 and TNF-α was determined.
Materials and Methods
It is a case-control study, comprising of 153 participants: 101 newborns with meningocele and 52 healthy newborns. The study was conducted in the Department of Biochemistry, Institute of Medical Sciences, Banaras Hindu University, Varanasi, in collaboration with the Department of Paediatric Surgery and Department of Obstetrics and Gynecology, Sir Sunderlal Hospital, Banaras Hindu University, Varanasi. The study was conducted during the period of 2012 to 2014.
Serum was extracted from blood collected from both groups i.e. meningocele patient group and healthy neonatal control group. The levels of MDA and SOD were determined by spectrophotometric method. IL-6 was determined by the Human IL-6 High Sensitivity ELISA Kit and TNF-α was determined by the Human TNF-α ELISA KIT.
Results
The levels of MDA, TNF-α and IL-6 were found to be much higher and level of SOD was found lower in the patients with meningocele as compared to the normal healthy neonates.
Conclusion
Increased MDA (oxidative damage product), IL-6, and TNF-α (inflammatory marker) and low level of SOD shows an increased inflammatory response in Meningocele. Our study shows Negative Correlation between MDA and SOD in case & control groups, while a Positive Correlation between TNF alpha and IL-6 in control & case groups.
Antioxidant, Congenital anomaly, Inflammatory marker, Newborn
Introduction
Meningocele (MM) is an abnormal growth of the meninges through a defect in the cranium or vertebral column. Anatomically meningocele is caused by the failure of the neural tube to close during the first month of embryonic development [1]. The process involves rostral and caudal closure of neural tube on 23rd and 27th day after fertilization respectively [2]. Studies have shown the probable role of oxidative stress and inflammation in the causation of oesophageal artesia which is also a congenital malformation found in neonates [3]. As for many diseases such as cancer, hypertension and atherosclerosis the genetic basis for meningocele is multi-factorial and also depends on environment [4].
There has been association between multivitamin use during the periconceptional period and the occurrence of neural tube defects. Mothers who were not using multivitamins any time during the six-month period, the chances of neural tube defect were more [5]. Folic acid deficiency is associated with neural tube defects such as meningocele and its incidence and severity reduces by 70% with folate supplementation [6–8].
Tumour necrotic factor (TNF) is a cytokine implicated in various diseases such as Alzheimer’s disease [9] cancer [10] and inflammatory bowel disease (IBD) [11]. It is a pro-inflammatory marker which is also a pyrogen, mediator of apoptosis, cachexia and sepsis. It is similar to IL-1 and IL-6 [12].
Superoxide is one of the main reactive oxygen species in the cell. Superoxide is produced as a by-product of oxygen metabolism and causes many types of cell damage. Superoxide dismutase (SOD) provides protection against oxidants and it is not expressed in developing foetus. It is also implicated in diseases such as Amyotrophic Lateral Sclerosis. Hence, high levels of free radicals can cause damage to them and induce congenital anomalies (neural tube defects) [13].
Malondialdehyde (MDA) is one of the products of lipid peroxidation which occurs mainly due to damaging effect of free radicals. MDA has been used for indirectly estimating the level of free radical generation in our body [14].
The aim of this study was estimation of the levels of MDA (Malonyldialdehyde), SOD (Superoxide dismutase) taken as oxidative damage markers and IL-6 (interleukin 6) and TNF-α (Tumour necrotic factor alpha) taken as inflammatory markers, in the serum of meningocele patients and age, sex matched normal neonates. Also, evaluate the causes of congenital anomaly in relation to biomarkers.
Materials and Methods
The study was conducted in the Department of Biochemistry, Institute of Medical Sciences, Banaras Hindu University, in association with the Department of Paediatric Surgery and Obstetrics and Gynecology, Sir Sunderlal Hospital, Banaras Hindu University, India.
Total 153 neonates were selected including 101 neonates with meningocele (confirmed by clinical examination and ultra sonography) 52 normal neonates taken as control. Mothers suffering from any acute infection or chronic diseases like diabetes mellitus, hypertension, and hyperlipidaemia or with any history of antiepileptic drug intake or iron, folic acid tablets intake during periconception period were identified and their neonates were excluded from the study in both cases and control. Mothers were physically normal at the time of clinical evaluation in Obstetrics and Gynecology OPD. Those neonates who were having infected meningocele were also excluded. The neonates (less than 28 days old) with meningocele included in our study were admitted in the Department of Paediatric Surgery after their clinical evaluation in the OPD. Our study only included the meningocele patients and meningomyelocele (meningocele with neural content) patients were excluded.
Informed consent was taken from all the mothers or legal attendants of each neonate enrolled in our study. Ethical clearance was taken from institution ethics committee before beginning of the study.
A 2 ml of venous blood was sampled from each subject under aseptic condition. Blood from the meningocele patients were taken after they being admitted to the neonatal ward and before any surgery was performed. The blood was allowed to stand for 30 minutes for spontaneous blood clotting. The serum was separated from the blood cells by centrifugation at 3000 rpm for 10 minutes at room temperature. The serum was taken out with pipette and centrifuged twice for 5 minutes at 3000 rpm to remove any blood cell remnants, pipette again, and then stored at −20°C in deionized eppendorf tube vials until assay.
All the chemicals and reagents required for the analysis were of analytical grade and proper aseptic measures were taken during the study. Estimation was done by Spectrophotometry (SL 164 double beam spectrophotometer). Malondialdehyde (MDA) is one of the products of lipid peroxidation. It can be estimated by the thiobarbituric acid (TBA) test. MDA reacts with TBA to generate a pink coloured product. In acid solution, the product absorbs light at 530 nm (in a spectrophotometer) [15]. MDA level was then calculated from the measured absorbance.
Superoxide dismutase (SOD) was assayed by the method of Marklund and Marklund [16]. This method is based on the ability of SOD to inhibit autoxidation of pyrogallol under specific conditions. Reading was taken at 420 nm in a spectrophotometer. SOD level was then calculated from the measured absorbance. Human IL-6 High Sensitivity ELISA KIT and Human TNF-α ELISA KIT (Diaclone SAS, F-25020, Besancon Cedex, France) were utilized in estimating the levels of IL-6 and TNF-α respectively in both cases and controls according to the instructions given in the instruction booklet of the kit.
Statistical Analysis
The current versions (2014) named IBM SPSS Statistics was used for statistical calculations. The data were expressed as mean ± S.D, p < 0.05 is considered significant and mean ± S.D, p < 0.0001 is highly significant. The results of cases and controls were compared with unpaired t-test for statistical analysis.
Results
In our study [Table/Fig-1] shows that mean serum MDA in meningocele patients and controls were 1.40±0.054 (μ mol/l) and (1.12±0.19 μ mol/l); mean SOD in patients 0.537±0.28 (U/ml) and in controls 1.08±0.087 (unit/ml). Pro- inflammatory marker IL-6 in patients and controls were 6.61±0.296(pg/ml) and 3.54±0.287(pg/ml); TNF-α in patients 29.34±0.32 (pg/ml) and in controls 22.5±0.153 (pg/ml). The serum lipid peroxide concentration in the form of MDA and the level of the antioxidant SOD both were significantly higher in meningocele patients (p > 0.001) as compared to that in the controls. The pro inflammatory markers in the form of TNF alpha and IL-6 both were significantly higher in meningocele patients (p > 0.001) as compared to that in the controls. [Table/Fig-2,3] shows the negative correlation between MDA and SOD while [Table/Fig-4,5] positive correlation between TNF alpha and IL-6.
Serum MDA, SOD, TNF alpha, IL-6 in control; group A (mean ± S.D, n =53 and cases; group B (n= 101 patients).
| S. No | Parameters | Control (Group A) | Cases (Group B) | p-value |
|---|
| 1. | MDA (μ mol/l) | 1.12±0.19 | 1.40 ±0.054 | <0.001 |
| 2. | SOD (unit/ml) | 1.08±0.087 | 0.537 ±0.28 | <0.001 |
| 3. | IL-6(pg/ml) | 3.54 ± 0.287 | 6.61± 0.296 | <0.001 |
| 4. | TNF-α (pg/ml) | 22.5 ± 0.153 | 29.34± 0.32 | <0.001 |
MDA –malondialdehyde; SOD -superoxide dismutase; IL-6-interleukin-6; TNF-α- tumour necrosis factor- alpha
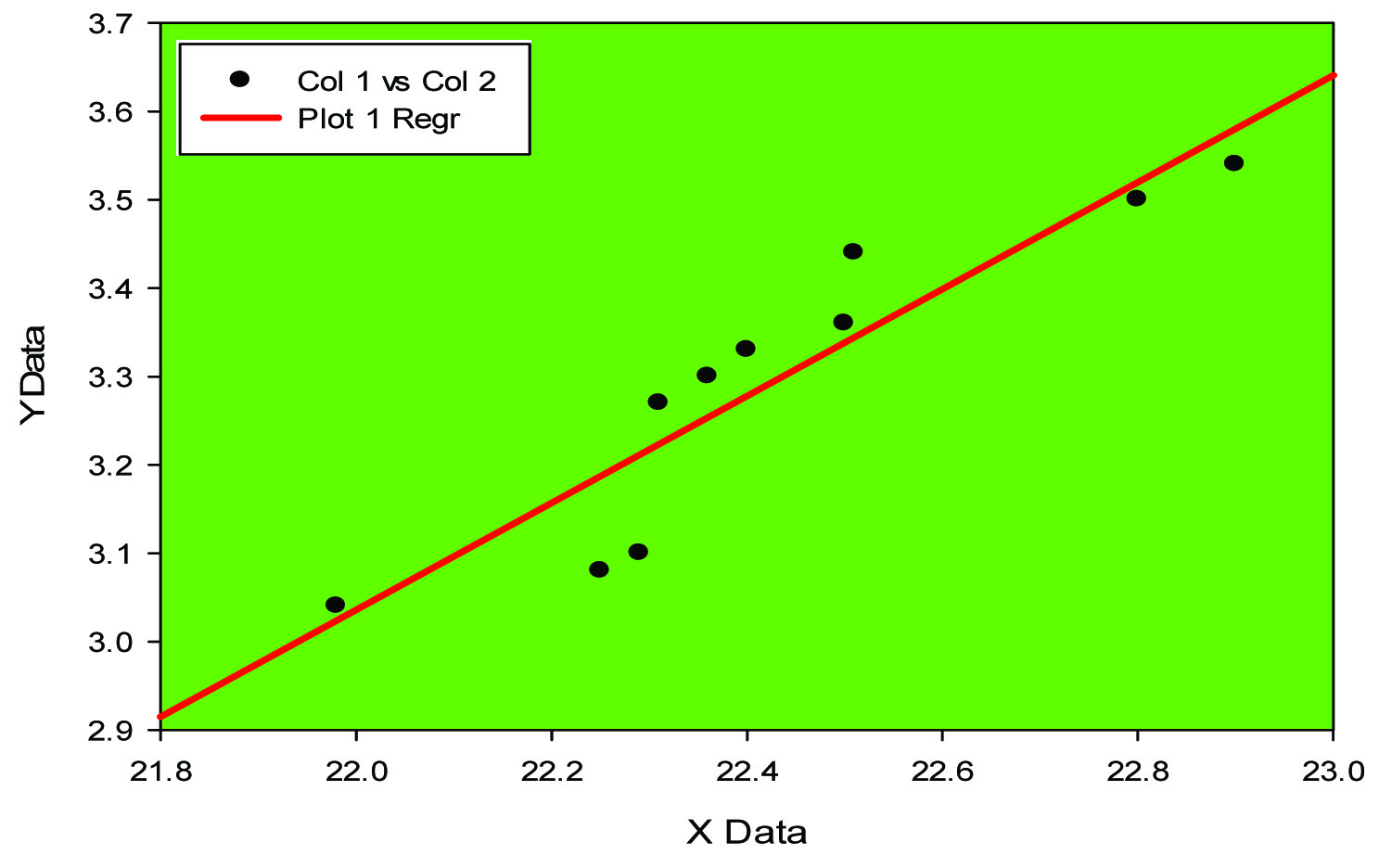
Positive Co-relation between TNF alpha and IL-6 in control (groupA)
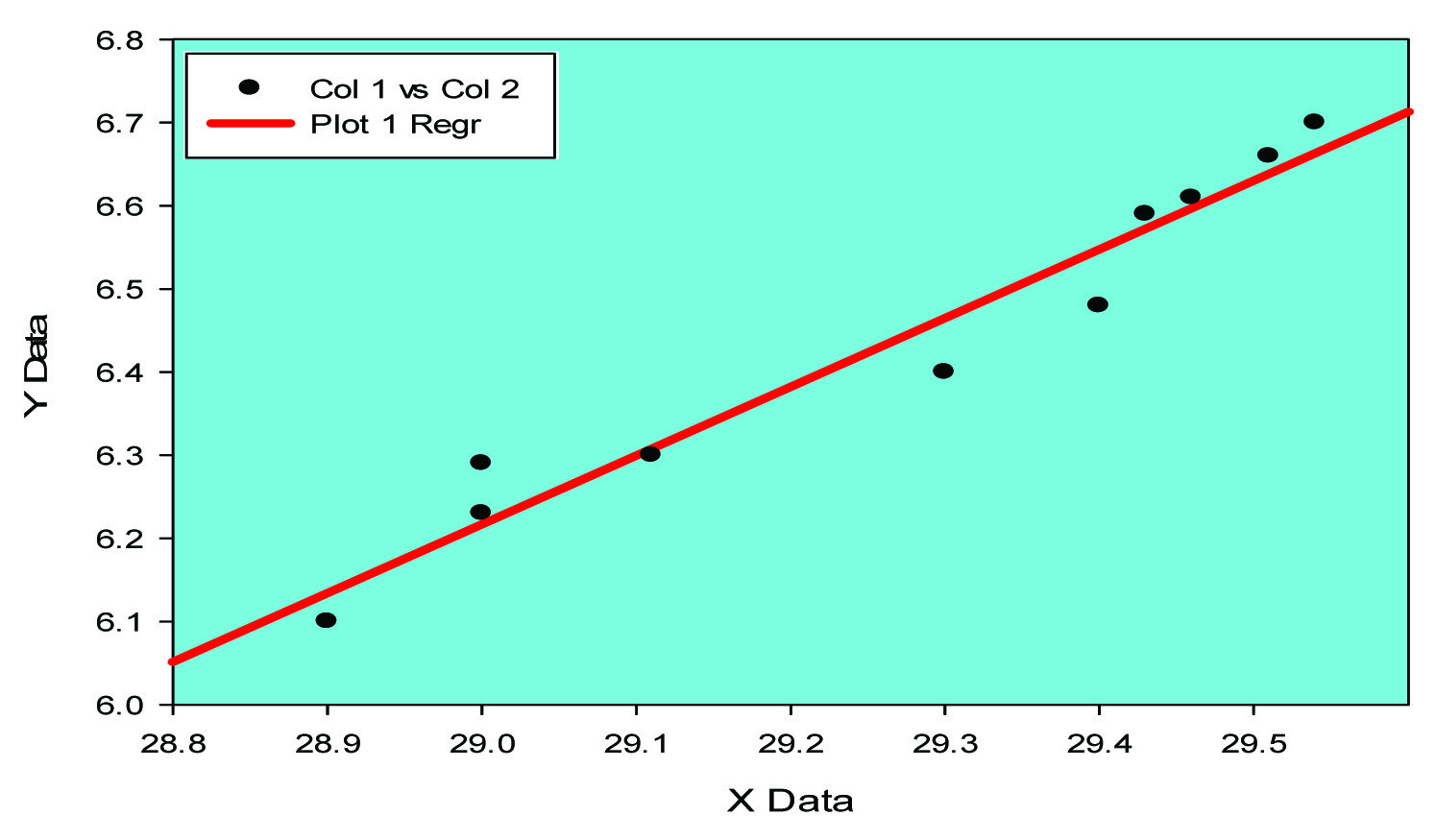
Positive Co-relation between TNF alpha and IL-6 in case (group B)
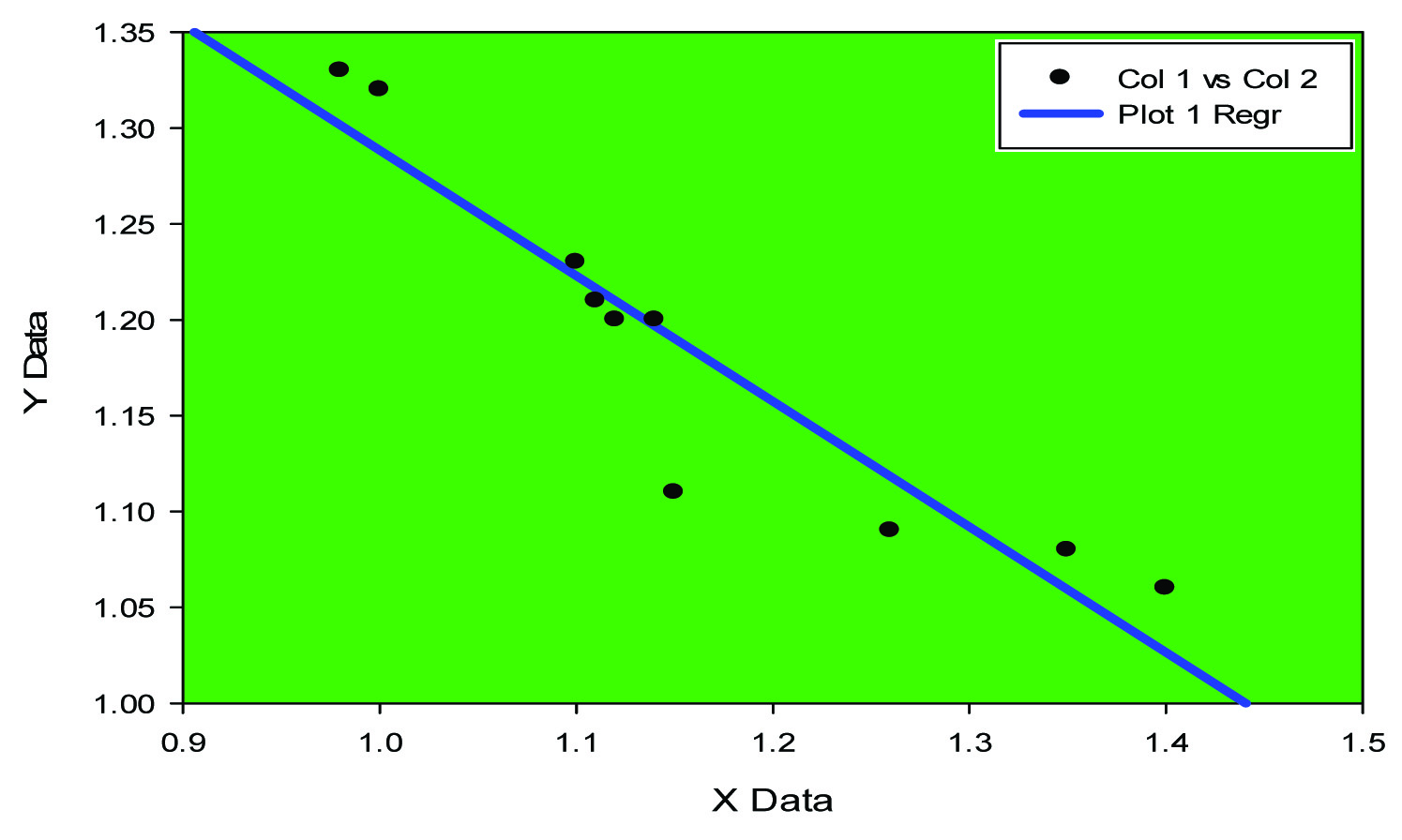
Negative Correlation between MDA and SOD in control (group A)
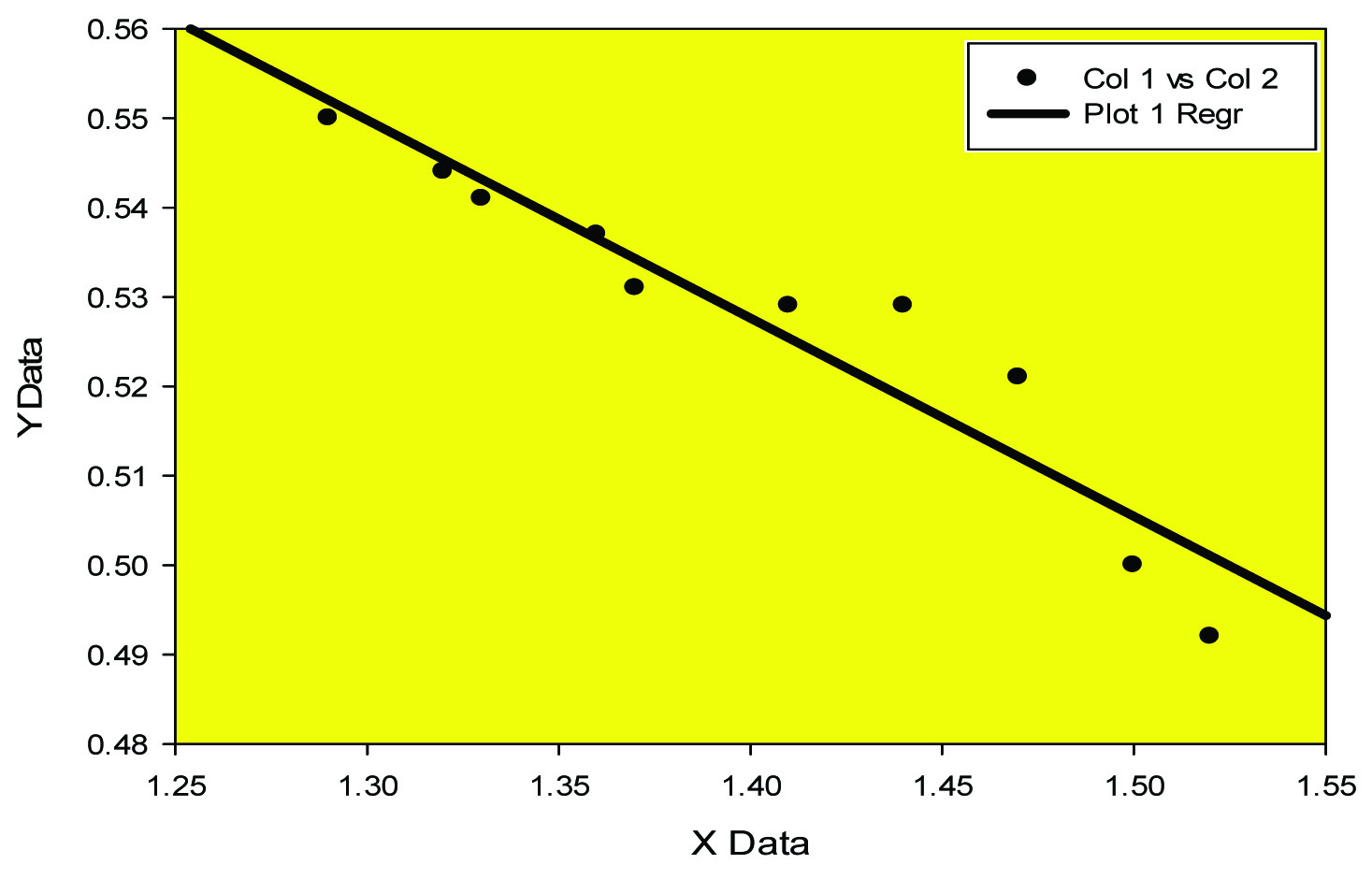
Negative Correlation between MDA and SOD in case (group B)
Discussion
Free radicals are generated in our body in healthy as well as in diseased conditions. Most cells receive approximately 10,000 free-radical hits a day. 95% of oxygen form the blood is used in the mitochondria for cellular respiration and 0.1% to 4% of the O2 is used actively by respiring mitochondria to form O2- (superoxide). Enzymes and antioxidant system can normally process these free radicals i.e. in health the free radicals are neutralized by the antioxidants. In disease either there is excess of free radical generation or there is reduced level of antioxidant in the body which cannot destroy the excess free radicals. This imbalance between free radical generation and destruction is known as oxidative stress [17].
In our study we used MDA as lipid peroxidation marker because MDA is a baseline free radical adduct from lipid peroxidation of plasma membrane lipids, SOD is a main antioxidant in our blood (mitochondrial and extra mitochondrial both) and IL-6, TNF alpha are the most common pro inflammatory markers in our body. For monitoring of lipid peroxidation MDA estimation is commonly used. The high level of MDA in our study was significant (p<0.001) that indicates the lipid peroxidation is taking place in patients of meningocele [Table/Fig-1]. Superoxide dismutase which nullifies the free radical superoxide is found to be higher in the patients (p<0.001), [Table/Fig-1].
Expression of cytokines like IL-6 and TNF-alpha has been elucidated in animal model (mice) [18]. In our study pro- inflammatory marker IL-6 has been found to be high [Table/Fig-1] (p<0.0001) along with high level of Tumour necrosis factor alpha (p<0.001), [Table/Fig-1]. Negative correlation between MDA and SOD [Table/Fig-2,3] while positive correlation between TNF alpha and IL-6 [Table/Fig-4,5] implicates the presence of inflammatory process and oxidative stress in meningocele
Conclusion
Interleukin 6 (IL-6) and TNF-α act as pro-inflammatory cytokines being secreted by T cells and macrophages. MDA being a marker of lipid peroxidation has been found to be of much higher level, showing presence of some ongoing inflammatory process. Similarly SOD which quenches free radicals is being shown to be of reduced level specifying the inability of the enzyme to nullify the effect of free radicals. So in conclusion it may be said that high levels of IL-6, TNF-α, MDA and low level of SOD in neonates with meningocele show, increased oxidative stress and excessive ongoing inflammatory reactions. As per clinical implication the outcome of treatment may be much better if oxidative stress and inflammation could be reduced.
MDA –malondialdehyde; SOD -superoxide dismutase; IL-6-interleukin-6; TNF-α- tumour necrosis factor- alpha
[1]. Kinsman SL, Johnston MV, Congenital anomalies of the central nervous system. In: Kliegman RM, Behrman RE, Jenson HB, Stanton BF, edsNelson Textbook of Pediatrics 2007 18th ed:592 [Google Scholar]
[2]. Lissauer T., Clayden G, Illustrated Textbook of Paediatrics (Second Edition) 2003 MosbyISBN 0-7234-3178-7 [Google Scholar]
[3]. Melek M, Demir H, Bilici S, Beger B, Çobanolu U, Meral I, Oxidative Stress and Antioxidant Enzyme Activities in Newborns with oesophageal Atresia and Their MothersJournal of International Medical Research 2012 40(1):249-57. [Google Scholar]
[4]. Centers for Disease Control and Prevention. (2011). Spina bifida: Facts. Retrieved March 30, 2012, from http://www.cdc.gov/ncbddd/spinabifida/facts.html [top] [Google Scholar]
[5]. Mulinare J, Cordero JF, Erickson JD, Berry RJ, Periconceptional use of multivitamins and the occurrence of neural tube defectsJAMA 1989 260(21):3141-45. [Google Scholar]
[6]. Mulinare J, Cordero JF, Erickson JD, Berry RJ, Periconceptional use of multivitamins and the occurrence of neural tube defectsJAMA 1988 260(21):3141-45. [Google Scholar]
[7]. Holmes LB, Does taking vitamins at the time of conception prevent neural tube defects?JAMA 1988 260(21):3181 [Google Scholar]
[8]. Milunsky A, Jick H, Jick SS, Multivitamin/folic acid supplementation in early pregnancy reduces the prevalence of neural tube defectsJAMA 1989 262(20):2847-52. [Google Scholar]
[9]. Swardfager W, Lanctôt K, Rothenburg L, Wong A, Cappell J, Herrmann N, A meta-analysis of cytokines in Alzheimer’s diseaseBiol Psychiatry 2010 68(10):930-41. [Google Scholar]
[10]. Locksley RM, Killeen N, Lenardo MJ, The TNF and TNF receptor superfamilies: integrating mammalian biologyCell 2001 104(4):487-501. [Google Scholar]
[11]. Brynskov J, Foegh P, Pedersen G, Ellervik C, Kirkegaard T, Bingham A, Tumour necrosis factor alpha converting enzyme (TACE) activity in the colonic mucosa of patients with inflammatory bowel diseaseGut 2002 51(1):37-43. [Google Scholar]
[12]. Kriegler M, Perez C, DeFay K, Albert I, Lu SD, A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNFCell 1988 53(1):45-53. [Google Scholar]
[13]. McCord JM, Fridovich I, Superoxide dismutase: the first twenty years (1968-1988)Free Radical Biology & Medicine 1988 5(5-6):363-9. [Google Scholar]
[14]. Devasagayam TPA, Boloor KK, Ramasarma T, Methods for estimating lipid peroxidation: an analysis of merits and demeritsIndian J Biochem Biophys 2003 40:300-08. [Google Scholar]
[15]. Satoh K, Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric methodClinica Chimica Acta 1978 90(1):37-43. [Google Scholar]
[16]. Marklund S, Marklund G, Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutaseEuropean Journal of Biochemistry 1974 47:469-74. [Google Scholar]
[17]. Nelson DL, Lehninger Principles of Biochemistry6th Edition:744-45. [Google Scholar]
[18]. Suganuma M, Okabe S, Kurusu M, Discrete roles of cytokines, TNF-α, IL-1, IL-6 in tumour promotion and cell transformationInternational Journal of Oncology 2002 :131-36. [Google Scholar]