Oral Squamous Cell Carcinoma (OSCC) is the sixth most common malignancy and is an important cause for cancer morbidity and mortality worldwide. An estimated five-year survival has remained approximately 50% despite the advanced treatment modalities available. A detrimental factor is the lack of early diagnosis and treatment intervention with a significant proportion of OSCCs remaining undiagnosed until they reach an advanced stage [1,2].
Saliva plays a pivotal role in the pathogenesis of OSCC and its composition has never been studied comprehensively in OSCC patients. Salivary analysis through assessment of its various components originating in the oral and oro-pharyngeal mucosa, as well as components originating in serum allows for the evaluation of local and systemic changes in the body. Salivary analysis can also be used to diagnose specific disease-related alterations such as epithelial tumour markers, which is an indicator of OSCC. Patients various salivary components thus represent both physiological and pathological alterations, thereby contributing to its diagnostic capabilities [3].
Lactate dehy drogenase (LDH) is a ubiquitous enzyme that catalyzes the reaction of lactate production via pyruvate reduction during anaerobic glycolysis. Within the cell, glucose is used principally for the production of pyruvate in the glycolysis pathway. Under aerobic conditions, pyruvate enters the mitochondrial matrix, where it is oxidized by the action of pyruvate dehydrogenase, being transformed into acetyl-CoA. LDH is an enzyme detectable in the cytoplasm of almost every cell in the human body, which becomes extracellular upon cell death. Therefore, its extracellular presence is always related to cell necrosis and tissue breakdown [4].
Its serum activity non-specifically increases in many pathological conditions such as myocardial infarction, liver disease (being particularly high in toxic hepatitis with jaundice), megaloblastic anemia’s, renal disease (especially in patients with tubular necrosis or pyelonephritis), malignant diseases (Hodgkin’s disease, carcinoma of the abdomen and lung, teratoma, liver metastases or leukaemia), progressive muscular dystrophy and pulmonary embolism.
Consequently, LDH concentration in saliva, as an expression of cellular necrosis, could be a specific indicator for oral lesions that affect the integrity of the oral mucosa. Furthermore, a possible correlation may exist between the levels of salivary LDH and the aggressiveness of oral OSCC lesion, as the mitotic rate of more aggressive lesions is higher and thus the salivary LDH is also expected to increase [4].
Many methods are available today for diagnosis of cancers but more emphasis is always given to a noninvasive and an accurate test for diagnosis, thus saliva an important and potential biomarker can be used as an adjunctive step for diagnosing oral cancers and precancers which improve the prognosis and outcome of the disease process [5]. The present study is thus an attempt to study the accuracy of salivary LDH as a potential biomarker for oral cancers detection and to determine the possible correlation between the levels of salivary LDH and tumour differentiation.
Materials and Methods
A comparative clinical study with 50 subjects was carried out. The study subjects were divided into two groups of Cases (including 30 patients) and Controls (including 20 subjects). The 30 subjects visiting the Department of Oral Medicine at the Bangalore Institute of Dental Sciences and the Department of Head and Neck Oncology at the various oncology centers in Bangalore, diagnosed histopathologically with oral squamous cell carcinoma, were selected for the study group comprising of both males and females aged between 35 to 65 years with a control group of 20 patients. Informed consent was taken from patients selected for the study.
Patients excluded from the study were those treated for cancers (including, surgery, chemotherapy, radiotherapy), patients with other systemic diseases known to probably increase serum LDH levels and with other oral conditions known to increase salivary LDH levels like periodontitis or mucosal lesions with tissue destruction [6,7].
Clinical Method- Patients selected were examined thoroughly and the findings related to their demographic status, sites, size of tumour were recorded in a case history annexure. These histopathologically diagnosed patients with oral squamous cell carcinoma were classified based on their grades of tumour differentiation into grades of well differentiated, moderately differentiated and poorly differentiated carcinoma respectively according to Broders grading of tumour differentiation [8]. Patients selected were subjected for ultrasonic scaling two weeks prior to sample collection, to rule out raised salivary LDH due to periodontitis.
Collection of Saliva- Patients were recalled after two weeks of ultrasonic scaling and reviewed. Unstimulated whole saliva was collected in a disposable and sterile plastic container by spitting method. Morning samples were preferred to avoid diurnal variations of salivary flow and changes in the sialochemistry. Patients were advised to avoid intake of water or food one hour prior to sample collection to avoid interference of food and water with the enzyme levels. Sample collected was immediately transported to the nearby laboratory in a span of 15 minutes and was immediately centrifuged and processed.
Laboratory Procedures- Levels of the enzyme activity in both control and study group were assessed through automated method using autoanalyser readings of enzyme activity at 37°C temperature and expressed in international units according to IFCC method [9].
The reagents selected for the study were based on the following catalytic reaction. Lactate dehydrogenase, a metabolic enzyme catalyses the reduction and conversion of the substrate pyruvate to lactate in the presence of NADH.

(Pyruvate is an end product of glycolysis which under anaerobic condition is reduced to lactate by the enzyme lactate dehydrogenase)
The rate of decrease in concentration of NADPH measured photometrically is proportional to the catalytic concentration of LDH present in the sample. Reagents thus used were Imidazole (concentration 65mmol/L) pyruvate as the buffer (concentration 0.6 mmol/L) and (NADH substrate) reagent 2 (concentration 0.18 mmol/L) supplied by SPINREACT COMPANY, SPAIN [9,10].
Four volumes of reagent 1 and one volume of regent 2 were mixed and the enzyme activity was observed using spectrophotometer at a wavelength of 340 nm (UV kinetic method).
Levels of the enzyme activity in both control and study group was tabulated and compared statistically using student t test; Similarly the levels of enzyme activity in the three subgroups of the study group were also compared using student t-test thus correlating tumour differentiation to levels of enzyme activity in saliva.
Results
A comparative clinical study with 50 subjects comprising of two groups namely the Cases (including 30 patients) and Controls (including 20 subjects). The 30 subjects taken as cases of squamous cell carcinoma were further subdivided into histopathological grades of well differentiated (15), moderately differentiated (10) and poorly differentiated carcinoma (5) respectively. Levels of the enzyme activity in both the control and the study group were compared and the levels of enzyme activity in the three subgroups of the study group were also compared. Mean+SD (Min-Max) was represented and statistical significance assessed using student t-test.
The highest LDH value obtained among the cases was 1835 U/L and the lowest value obtained was 925 U/L. The highest value of LDH among the control group was 587U/L and the lowest value obtained was 418U/L. The controls showed a mean value of 497.00 with a SD of 51.75 where as the cases showed a mean value of 1225.40 with a SD of 221.79. The p-value calculated was 0.0001 and the results thus obtained were statistically significant [Table/Fig-1].
Mean and standard deviation of cases and controls student t-test.
| Case (LDH Value) | Control (LDH Value) |
|---|
| Mean | 1225.40 | 497.00 |
| SD | 221.79 | 51.75 |
| p-value | 0.0001 (Significant) |
When the LDH values for the various grades were compared the mean values were 1049.07, 1309.50 and 1586.20 respectively for well differentiated, moderately differentiated and poorly differentiated carcinoma and their SD was 46.89, 68.79 and 203.20. The p-value obtained was as follows: Well differentiated carcinoma versus Moderately differentiated carcinoma (p=0.0001) Well differentiated carcinoma versus Poorly differentiated carcinoma (p=0.0003), Moderately differentiated carcinoma versus Poorly differentiated carcinoma (p=0.0036) this showed that the LDH values significantly correlated with the histopathological grade of the tumour [Table/Fig-2].
Mean and standard deviation of the grades of oscc student t-test
| Well Diff ca (LDH) | Mod Diff ca (LDH) | Poor Diff ca (LDH) |
|---|
| Mean | 1049.07 | 1309.50 | 1586.20 |
| SD | 46.89 | 68.79 | 203.20 |
| Well (LDH) VS Mod (LDH) | Well(LDH) VS Poor (LDH) | Mod(LDH) VS Poor(LDH) |
| p-value | 0.0001 (Significant) | 0.0003 (Significant) | 0.0036 (Significant) |
Discussion
OSCC is the most common cancer affecting the oral cavity. The poor prognosis is likely due to several factors including late diagnosis of OSCC. Thus early diagnosis is the most important factor that is directly related to successful treatment and likely cure.
One approach to this problem would be to improve the ability of oral health care professionals to detect relevant potentially malignant lesions or cancerous lesions at their earliest or most incipient stage. Such a goal could be achieved by increasing public awareness about the importance of regular oral screening or case finding examinations to identify small, otherwise asymptomatic cancers and precancers [5].
Another strategy would be the development of routine screening procedures, patient education and the use of diagnostic aids such as illuminating tools (velscope), tumour markers in serum such as anti-p53 antibody and markers in saliva such as DNA, RNA, and protein derived from the living cancer cells [11].
Saliva plays a very important role in diagnosis of several oral diseases. This property of saliva is attributed to variation in its constituents and composition [12,13]. Salivary analyses as an alternative approach to serological investigations has proven to be an effective and a non invasive investigation with emerging trends in various aspects of diagnosing several oral diseases [14].
Lactate dehydrogenase, a metabolic enzyme in the anaerobic glycolysis is present in the cytoplasm of all living normal cells which becomes extracellular upon cell death. Therefore, its extracellular presence is always related to cell necrosis and tissue breakdown [15].
Raised serum levels of the enzyme are observed in several other systemic conditions including malignancies, myocardial infarction, liver disease, megaloblastic anemia’s, renal disease and periodontal disease therefore its raised levels in serum is not specific [15]. These conditions were listed as exclusion criteria in our study to avoid obtaining a false result. Saliva, an alternative to serum can be used as a screening test for diagnosis of oral malignancies. Biomarkers in saliva are proven to be raised in oral carcinomas. Thus, presence of increased levels of these biomarkers is a non specific indicator of the disease process [16].
Our study was aimed to obtain an easily performable test that can be carried out with minimal discomfort to the patients providing accurate results. Saliva, the oral fluid with its enormous diagnostic properties was considered an appropriate medium to aid our study [17].
When salivary LDH values were compared among the 30 cases of OSCC and 20 normal controls it was found that the LDH values in the 30 cases were significantly higher than in the controls (p = 0.0001). The p-value obtained was statistically significant which proved our first objective of using salivary LDH as a potential biomarker for OSCC detection and diagnosis [Table/Fig-3].
LDH values for cases versus controls.
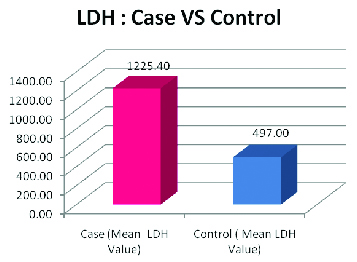
These results were in accordance with the study conducted by Shpitzer T et al., who utilized comprehensive salivary analysis to evaluate biochemical and immunological parameters in the saliva of (OSCC) patients and found that in cancer patients, salivary concentration of LDH was significantly higher by 88% [18].
In another study carried out by Nagler et al., showed a profound increase in salivary LDH which is known to be mainly derived from the exfoliative oral epithelial cells (in this case OSCC cells) and as such may also be used as a general salivary marker for the diseased mucosa [15].
Nagler et al., also stated about a future interesting prospective of salivary LDH study to examine the correlation between the level of salivary LDH and the aggressiveness of oral OSCC lesion, as the mitotic rate of more aggressive lesions is higher and thus the salivary LDH may also be expected to increase [15]. “This hypothesis was also put to test in our study”.
Several studies have been performed correlating levels of LDH enzyme activity in serum to the differentiation of tumour and have demonstrated a significant correlation between varied levels of the enzyme to the aggressiveness of the tumour. Perhaps, ours is the first study carried out by using saliva for determining the aggressiveness of tumour, since no English literature reports this kind of study for determining aggressiveness of oral cancers.
When the LDH values for the various grades of OSCC were compared and analysed statistically by Student t-test it was seen that the p-value obtained was statistically significant and the LDH values significantly correlated with the histopathological grade of the tumour [Table/Fig-4].
Mean LDH value for the grades of oral squamous cell carcinoma.
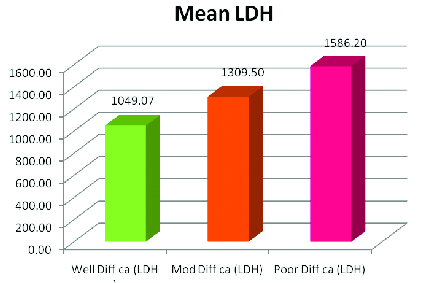
Our results were in accordance with the study conducted by MI Koukourakis, Giatromanolaki; E Sivridis who observed an over expression of LDH activity in serum in the advanced cancers of 76 patients with lung cancer [19]. They concluded that expression of this biochemical marker in serum can predict the aggressiveness of tumour and thus the prognosis. In another study by Mall et al., it was found raised serum LDH correlated with the advanced grades in small cell cancers of the lungs [20].
However, both these studies were carried out using serum, unlike in our study where we used saliva as a non invasive diagnostic medium to establish the same.
This current study was framed with a concept of establishing a non invasive, easily available, screening test for oral squamous cell carcinoma using a salivary marker.
A significant difference in the salivary LDH values was observed between the cases of OSCC and the control group. Furthermore, it was also observed a significant correlation of the enzyme levels with the differentiation of the tumour, thus predicting prognosis and the overall treatment outcome. Thus, metabolic alterations of saliva could be both an epidemiological marker and a target for chemoprevention of oral and oropharyngeal carcinogenesis.
The significant increase in salivary enzyme levels in patients with OSCC observed in our study reveals advantages of saliva measurement in comparison with serum analysis.
Though salivary diagnosis aids in preliminary detection of OSCC, a definitive diagnosis is always based on a biopsy and histopathological examination which remains as the gold standard.
Conclusion
The increase in salivary enzyme levels may be used as a diagnostic tool, especially when a concurrent analysis for significantly increased markers is carried out. This is because salivary diagnosis involves a noninvasive method, and may thus represent an effective alternative to serum testing. Though the definitive diagnosis of OSCC is based on a biopsy and histopathological examination, it would be highly desirable and beneficial if salivary tumour marker analysis could be done on a routine basis between biopsies.