Non-Functional Paraganglioma of the Urinary Bladder Treated by Transurethral Resection: Report of Two Cases
Richa Katiyar1, Saloni Dwivedi2, Sameer Trivedi3, Shashikant C.U. Patne4, Uday Shankar Dwivedi5
1 Service Senior Resident, Department of Pathology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, (U.P.), India.
2 Junior Resident, Department of Pathology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, (U.P.), India.
3 Associate Professor, Department of Urology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, (U.P.), India.
4 Assistant Professor, Department of Pathology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, (U.P.), India
5 Professor and Head, Department of Urology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, (U.P.), India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Shashikant C.U. Patne, Assistant Professor, Department of Pathology, Institute of Medical Sciences, Banaras Hindu University, Varanasi-221005, (U.P.), India. E-mail : scup.pathology08@gmail.com
Paraganglioma of the urinary bladder is a rare tumour derived from chromaffin tissue of the sympathetic nervous system. Paraganglioma of the urinary bladder especially the non-functional type is often misdiagnosed as urothelial cancer. Two female patients aged 32 years and 45 years presented with painless haematuria without any symptoms of catecholamine excess. Radiological investigations revealed urinary bladder tumour. The tumour was removed by transurethral resection in both the patients. Histopathological diagnosis was paraganglioma, which was confirmed by immunohistochemistry. Complete resection of tumour by transurethral approach is curative in paraganglioma of the urinary bladder. We hereby, also discuss the salient features of nonfunctional paraganglioma of the urinary bladder.
Catecholamines, Chromogranin, Neuroendocrine, Pheochromocytoma
Case Reports
Case 1
A 32-year-old female presented with painless gross haematuria and passage of blood clots since two months. Her blood pressure was 120/78 mm Hg. Except for the pallor, her general and systemic examinations were unremarkable. Her ultrasonography of the pelvis revealed a solid hypervascular hypoechoic lesion of approximately 2.9×2.3 cm size at the vesico-ureteric junction [Table/Fig-1a]. Her CT scan of the lower abdomen showed a 2.6×2.4 cm sized mildly enhancing soft tissue density lesion in the posterior wall of the urinary bladder near the left vesico-ureteric junction [Table/Fig-1b]. A 7.4×6.02 cm sized blood clot was also seen [Table/Fig-1b]. With a provisional clinical diagnosis of carcinoma of the urinary bladder, a Transurethral Resection of the Bladder Tumour (TURBT) was done, blood clot was removed, and tissue was submitted for histopathological examination. Microscopy showed a bit of unremarkable urothelial lining along with many bits of tumour arranged in zellballen pattern and surrounded by slender fibrovascular septae [Table/Fig-2a]. The individual tumour cells were polygonal with stippled chromatin, inconspicuous nucleoli, and moderate amount of granular cytoplasm. In few areas, tumour cells with clear cytoplasm were also evident. Mitotic figures were not seen. Immunohistochemical staining showed strong cytoplasmic positivity of chromogranin-A [Table/Fig-2b] and neuron specific enolase in the tumour cells, while sustentacular cells were highlighted by S-100 protein [Table/Fig-2c]. Pancytokeratin expression in the underlying tumour nests was negative. Ki-67 labeling index was <1%. Overexpression of Vascular Endothelial Growth Factor (VEGF) by immunohistochemistry was not seen in the tumour cells. Final diagnosis was non-functional paraganglioma of the urinary bladder. Patient did not receive any further treatment. On a recent follow-up at 10 months, her cystoscopy examination did not reveal any evidence of recurrence. Her ultrasound examination of the abdomen was negative for metastatic disease.
Case 2
A 45-year-old female presented with painless haematuria of two months duration. Her general and systemic examinations were unremarkable. Her blood pressure was 118/84 mm Hg. Her hemoglobin was 10.8 gm/dl, while rests of the investigations were within the normal limits. Her urinalysis was remarkable for 60-80 red blood cells/hpf and positive (3+) test for occult blood. Her ultrasonography revealed a 3.4×2.3 cm size hypoechoic hypervascular solid mass lesion in the anterior wall of the urinary bladder. Her CT scan demonstrated a homogenously enhancing polypoidal mass lesion of approximately 2.4×2.2×2.6 cm in antero-posterior, transverse and cranio-caudal axis, respectively arising from the anterior wall of the urinary bladder. There was no evidence of calcification within the lesion. Significant enlargement of regional or distant lymph nodes was not seen. Provisional radiological and clinical diagnosis was bladder carcinoma. TURBT was done while separate biopsy was taken from the superficial and deep muscle tissue of the urinary bladder. Microscopic examination showed part of unremarkable urothelium with underlying nests of tumour arranged in zellballen pattern and surrounded by thin fibrovascular septa. Individual tumour cells showed round to oval nuclei, stippled chromatin, inconspicuous nucleoli, and moderate amount of eosinophilic granular cytoplasm. Necrosis, mitotic figures and nuclear atypia were not seen. Immunohistochemistry of the tumour was positive for chromogranin-A, and neuron specific enolase, while sustentacular cells stained positive by S-100 protein. Pancytokeratin expression was observed in urothelial lining, underlying tumour nests were negative [Table/Fig-2d]. Ki-67 labeling index was <1%. Overexpression of VEGF by immunohistochemistry was not seen in the tumour cells. Superficial and deeper muscle biopsy was free of tumour infiltration. The final diagnosis was non-functional paraganglioma of the urinary bladder. In a follow-up of 6-months, patient was free of recurrence and metastasis by cystoscopy and abdominal ultrasound examinations, respectively.
a) Ultrasound examination showing hypoechoic mass in the urinary bladder; (b) CECT scan showing posteriorly located contrast enhanced polypoid mass (arrow) and a large blood clot (asterisk) in the urinary bladder.

(a) Showing zellballen pattern of tumour (H&E stain, 400×); (b) Strong immunoreactivity of chromogranin-A in the tumour cells (DAB, 400×); (c) Sustentacular cells of the tumour highlighted by S-100 protein (DAB, 400×); (d) Urothelial lining showing strong pancytokeratin immunoreactivity (arrow), while underlying nests of the tumour is negative (DAB, 100×).
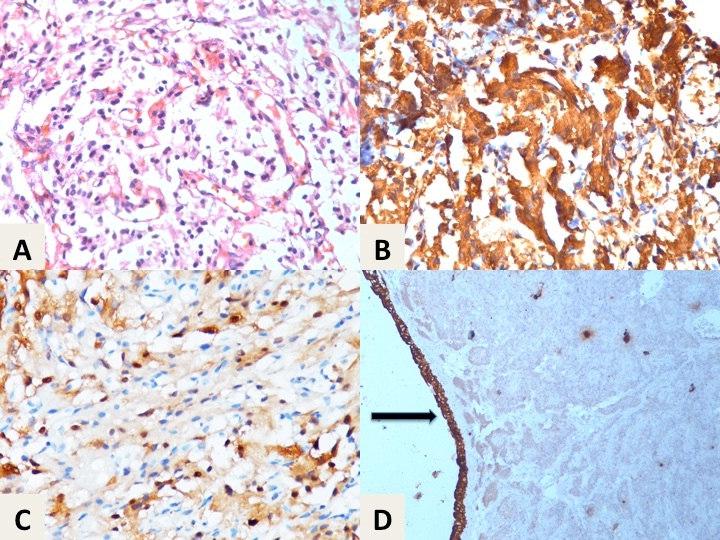
Discussion
Paraganglioma or extra-adrenal pheochromocytoma is a rare tumour that is derived from chromaffin tissues of the sympathetic nervous system [1]. Occurrence of paraganglioma in the urinary bladder is extremely rare, which constitutes less than 1% of all urinary bladder tumours and 6% of all paragangliomas [2]. In 1953, Zimmerman et al., reported the first ever case of urinary bladder paraganglioma (UBP) in a 74-year-old woman [3]. Since then, more than 200 cases of this rare entity have been reported [4]. Most cases of UBP occur at the age of 20-40 years and are more common in females than males [1]. These tumours are mostly seen at trigone and posterior wall of the urinary bladder [5].
Based on catecholamine secretion, UBP is classified into functional and non-functional types. Majority of the UBP are functional type and secrete catecholamine resulting in symptoms such as paroxysmal hypertension, palpitation, micturitional syncope, and headache [1]. Non-functional types constitute only about 17% of all UBP, lack symptoms of catecholamine excess and, therefore, often misdiagnosed as urothelial cancer during pre-operative evaluation [6,7]. We report two cases of non-functional UBP, which were misdiagnosed as urothelial cancer. The probability of UBP to be misdiagnosed as urothelial cancer is more due to its rarity, invasion of the muscle layer, morphology suggesting urothelial cancer in TURBT specimens and failure of pathologist to include paraganglioma in the differential diagnosis [7]. Whenever paraganglioma is suspected, tests for urinary vanillyl mandelic acid should be performed in 24-hour urinary sample. However, in the absence of characteristic symptoms of catecholamine excess, non-functional UBP is more likely to be misdiagnosed as urothelial cancer. This has actually happened in both of our patients, who presented with haematuria without any history of hypertension, headache, or flushing, which would indicate a diagnosis of paraganglioma. In a recently reported non-functioning UBP, fluctuant blood pressure and tachyarrhythmia was noted during TURBT procedure [5]. Conversely, in our case blood pressure at the time of admission as well as during the procedure of TURBT was within the normal limits. Therefore, diagnosis of paraganglioma was not suspected and so, investigations for catecholamine metabolites were not done in both the patients.
The pathogenesis of UBP is not clear until date. Although, it has been proposed that UBP originates from autonomic ganglia of the urinary bladder wall [1]. Cystoscopic appearance of a submucosal yellow tumour should raise the suspicion of UBP [5]. The correct preoperative diagnosis of UBP is often difficult; definite diagnosis is achieved on histopathology and immunohistochemistry of the excised tumour. Histopathologically, the tumour cells show characteristic zellballen or nesting pattern with delicate fibrovascular stroma [7]. Immunohistochemistry shows strong cytoplasmic expression of chromogranin, synaptophysin and neuron specific enolase in the tumour cells whereas supporting cells stain by S-100 protein [4]. The differential diagnoses of the UBP include urothelial carcinoma, metastatic renal cell carcinoma, prostatic carcinoma, malignant melanoma, and granular cell tumour [7]. Histological appearance and immunoprofile as described above helped us in distinguishing UBP from other differential diagnoses.
Treatment options for UBP include transurethral resection, partial cystectomy and radical cystectomy. Partial cystectomy is said to be the treatment of choice for UBP [8]. However, occasional report suggests successful treatment of UBP by transurethral resection [9]. We have successfully treated both the cases of UBP by TURBT. Thus, complete resection of tumour by transurethral approach is curative and obviates the need for partial or radical cystectomy in patients of UBP.
Since UBP is a rare tumour with uncertain biological behavior, its prognosis is not well established [6]. Most of the cases of UBP are benign while about 10-15% is malignant [2]. Abnormal vessel architecture and high expression of Vascular Endothelial Growth Factor (VEGF) in the tumour cells predicts malignant behavior of UBP [4]. Paraganglioma in both of our patients did not show either abnormal vessels or overexpression of VEGF by immunohistochemistry. However, the only definite evidence of malignancy in paraganglioma is invasion of the tumour to the adjacent organs or distant metastasis [10]. The rate of local recurrence varies from 5-15% and therefore, long-term follow up is necessary [10]. Both of our patients are under regular follow-up without any evidence of tumour recurrence or metastasis at the last follow-up.
Conclusion
We have reported two cases of non-functional paraganglioma of the urinary bladder, which did not show any symptoms of catecholamine excess and clinically mimicked with urothelial cancer. Transurethral resection of the bladder tumour has served both diagnostic and therapeutic purposes. Histopathological examination and immunohistochemistry is mandatory for diagnostic confirmation. Regular follow-up by cystoscopy examination is required to rule out recurrence in these patients.
[1]. Deng J-H, Li H-Z, Zhang Y-S, Liu G-H, Functional paragangliomas of the urinary bladder: a report of 9 casesChin J Cancer 2010 29:729-34. [Google Scholar]
[2]. Pastor-Guzmán JM, López-García S, Giménez-Bachs JM, Ruíz-Mondejar R, Cañamares-Pabolaza L, Atiénzar-Tobarra M, Paraganglioma of the bladder: controversy regarding treatmentUrol Int 2004 73:270-75. [Google Scholar]
[3]. Zimmerman IJ, Biron RE, MacMahon HE, Pheochromocytoma of the Urinary BladderN Engl J Med 1953 249:25-26. [Google Scholar]
[4]. Kovacs K, Bell D, Gardiner GW, Honey RJ, Goguen J, Rotondo F, Malignant paraganglioma of the urinary bladder: Immunohistochemical study of prognostic indicatorsEndocr Pathol 2005 16:363-69. [Google Scholar]
[5]. Gupta V, Sharma J, Sangwaiya A, Kaira V, Samal S, Sen R, Nonfunctioning paraganglioma of the urinary bladder: A rare entityClin Cancer Investig J 2015 4:268-70. [Google Scholar]
[6]. Lai Y, Chen D, Yu Z, Ni L, Yang S, Non-functioning paraganglioma of the urinary bladder: A case report and review of the literatureOncol Lett 2014 7:891-93. [Google Scholar]
[7]. Zhou M, Epstein JI, Young RH, Paraganglioma of the urinary bladder: a lesion that may be misdiagnosed as urothelial carcinoma in transurethral resection specimensAm J Surg Pathol 2004 28:94-100. [Google Scholar]
[8]. Xu D-F, Chen M, Liu Y-S, Gao Y, Cui X-G, Non-functional paraganglioma of the urinary bladder: a case reportJ Med Case Rep 2010 4:216 [Google Scholar]
[9]. Baima C, Casetta G, Vella R, Tizzani A, Bladder pheochromocytoma: a 3-year follow-up after transurethral resection (TURB)Urol Int 2000 65:176-78. [Google Scholar]
[10]. Wu P, Ho H, Yang C, Chen C, Non-functioning paraganglioma of the urinary bladderMid Taiwan J Med 2009 14:46-49. [Google Scholar]