Psoriasis is a chronic inflammatory auto-immune skin disease affecting 1-3% of the population [1,2]. Psoriasis predominantly affects the skin with scaly plaques involving mainly the extensor surfaces of the body like scalp, back, elbows, knees etc. Chronic plaque psoriasis is the most common morphological form. Due to the role of auto-immune mechanisms in the pathogenesis of psoriasis, it is regarded as a systemic disease [3]. Recently, a number of co-morbidities have been described in psoriasis patients, including cardiovascular co-morbidities, metabolic syndrome and malignancies [4,5]. Hypertension, heart failure and diabetes mellitus have been found to be more common among psoriasis patients [6].
Metabolic syndrome is a group of risk factors for cardiovascular disease and diabetes consisting of raised blood glucose level, increased blood pressure, high triglyceride levels, low high density lipoprotein cholesterol (HDL) levels and central obesity [7]. The association of metabolic syndrome with psoriasis could be due to higher prevalence of cigarette smoking, obesity, physical inactivity, hyperhomocysteinemia and psychological stress among patients [8]. Moreover hypertension, dyslipidaemia, insulin resistance and obesity are independently related to psoriasis other than as components of metabolic syndrome [9–11].
The association between psoriasis and metabolic syndrome has been studied with varying results. Furthermore, there is insufficient information regarding the relationship between the duration and severity of psoriasis with the development of metabolic syndrome. Psoriasis patients present early as the skin lesions are highly visible. On the other hand, metabolic syndrome is associated with insidious changes and late symptoms resulting in late detection. However, metabolic syndrome caries a high burden of morbidity as well as mortality. The association of these two disorders presents early opportunities of diagnosing and treating metabolic syndrome in psoriasis patients which could lead to a substantial reduction in the morbidity and mortality due to non-communicable diseases.
The characteristics of both psoriasis and metabolic syndrome show a marked variation among different geographic locations and ethnic origins. There is a substantial knowledge gap and paucity of data about the complex interplay between psoriasis and metabolic syndrome in a coastal, south-Indian population. Ideally a large population based, clinico-epidemiological study would help to bridge this gap. However, this design is difficult to achieve in our setting due to constraints of time and finance. Thus a hospital based case-control study design is a feasible alternative commensurate with the time limit, available funding and patient compliance.
A hospital-based case-control study was thus conducted to measure the association between psoriasis and metabolic syndrome in a south-Indian coastal population. The study also aimed to find out any correlation between the duration of the disease and occurrence of metabolic syndrome.
Materials and Methods
The study is a hospital-based case-control study carried out in the Dermatology out-patients department (OPD) and Biochemistry clinical laboratory at a medical college in Pondicherry. The study was approved by the institutional research committee and ethics committee which follows the guidelines set by the Helsinki declaration.
Thirty clinically diagnosed adult patients of chronic plaque psoriasis and 30 age- and sex-matched control subjects were included in the study. The control subjects were taken from patients attending the dermatology OPD for disorders other than psoriasis. Patients and controls currently on systemic steroids, immunosuppressants and systemic retinoids were excluded from the study. Informed consent was taken from both cases and control subjects. Detailed epidemiological information along with a detailed history was taken from the patients and hospital records. Specific emphasis was given to age, gender, and history of smoking and alcohol intake, duration and severity of disease, history of treatment if any, exacerbations and remissions and other co-existing medical conditions. General physical examination was done with special emphasis on height, weight, body mass index {calculated by the formula: weight (in kg)/ (height in m)2}, waist circumference and blood pressure. A detailed cutaneous examination including the type, distribution and arrangement of primary lesions and secondary changes in patients were undertaken. After overnight fasting, venous blood samples were collected from the subjects under complete aseptic precautions and were analysed for serum glucose, triglyceride and HDL-cholesterol in the clinical Biochemistry laboratory. The serum samples were analysed by using fully-automated clinical chemistry analyser (Roche Cobas Integra-400+) using kits supplied by Roche Diagnostics according to manufacturers’ protocol. Serum glucose was estimated by enzymatic reference method using hexokinase, serum triglycerides by enzymatic colorimetric method (GPO/PAP) with glycerol phosphate oxidase and 4-aminophenazone and serum HDL-cholesterol (HDL-c) by homogeneous enzymatic colorimetric assay [12–14].
The diagnosis of metabolic syndrome (MS) was based on the criteria of National Cholesterol Education Program – Adult Treatment Plan III, with Asian modification for abdominal circumference [7]. The criteria are:
i) Increased waist circumference – In Asian populations, ≥ 90 cm in males and ≥ 80 cm in females;
ii) Raised triglycerides ≥150 mg/dl (or on treatment for raised triglycerides);
iii) Decreased HDL < 40 mg/dL in men and < 50 mg/dL in women (or on treatment for reduced HDL-c);
iv) Increased blood pressure systolic ≥130 and/or diastolic ≥ 85 mm Hg (or on treatment for hypertension);
v) Increased fasting glucose ≥ 100 mg/dL (or on treatment for increased blood glucose).
The presence of any 3 or more of these 5 risk factors constitutes a diagnosis of MS [7].
Statistical Analysis
The data were systematically arranged and analysed by SPSS software version 16 and p-value < 0.05 was considered significant. For normally distributed data, Students t-test was done to calculate statistical significance. For parameters whose distribution was skewed, Mann-Whitney U test was done for statistical significance. To detect statistically significant difference between the percentages of cases and controls testing positive for a particular parameter, chi-square test was done.
Results
There was no statistically significant difference in the mean age and BMI between cases and controls [Table/Fig-1]. The proportions of males and females were also not significantly different between cases and controls, achieving age and sex-matching of cases and controls [Table/Fig-2]. The difference between the percentages of cases and controls with history of smoking and alcohol intake was statistically not significant either [Table/Fig-2].
Distribution of age and body mass index (BMI) among cases and controls.
| Parameter | Controls | Cases | p-value |
|---|
| Mean | SD | Mean | SD |
|---|
| Age (years) | 45.77 | 12.09 | 42.07 | 12.58 | 0.25 |
| BMI | 24.56 | 4.76 | 24.23 | 3.91 | 0.77 |
The values are for 30 cases and 30 controls. The differences are not statistically significant (p-value >0.05) as calculated by student’s t-test
Distribution of sex, history of smoking and history of alcohol intake among cases and controls.
| Parameter | Controls | Cases | p-value |
|---|
| Number (out of 30) | Percentage (%) | Number (out of 30) | Percentage (%) |
|---|
| Sex (no. of females) | 15 | 50 | 13 | 43.3 | 0.61 |
| History of smoking | 3 | 10 | 5 | 16 | 0.72 |
| History of alcoholic intake | 12 | 40 | 15 | 50 | 0.54 |
The values are for 30 cases and 30 controls. The differences are not statistically significant (p-value >0.05) as calculated chi-square test.
The data presented in [Table/Fig-3] represents the mean values of the different parameters of metabolic syndrome viz. fasting serum glucose (FBS), fasting HDL-c, waist circumference (WC) and blood pressure of both cases and controls. The differences between the means of the 2 groups were statistically not significant for all the parameters. In case of serum triglycerides (TG), the data had a skewed distribution and so the median and the inter-quartile range have been considered [Table/Fig-4]. There was no statistically significant difference between the two medians.
Comparison of different parameters of MS (except TG) between cases and controls.
| Parameter | Controls | Cases | p-value |
|---|
| Mean | SD | Mean | SD |
|---|
| FBS (mg/dL) | 134.17 | 60.04 | 118.40 | 41.78 | 0.243 |
| Serum HDL-c (mg/dL) | 38.93 | 10.53 | 37.77 | 11.03 | 0.677 |
| Systolic BP (mm of Hg) | 122.86 | 14.97 | 121.66 | 14.85 | 0.765 |
| Diastolic BP (mm of Hg) | 80.86 | 11.68 | 78.88 | 12.67 | 0.645 |
| WC (cm) | 89.28 | 11.77 | 85.36 | 8 | 0.137 |
The values are for 30 cases and 30 controls. The differences are not statistically significant (p-value >0.05) as calculated by student’s t-test.
Comparison of serum TG between cases and controls.
| Parameter | Controls | Cases | p-value |
|---|
| Median | Range | Median | Range |
|---|
| Serum TG (mg/dL) | 120.5 | 73.75, 169 | 145 | 106, 203.25 | 0.102 |
The values are for 30 cases and 30 controls. The differences are not statistically significant (p-value > 0.05) as calculated by Mann-Whitney U test.
[Table/Fig-5] represents the percentages of cases and controls fulfilling each of the criteria of National Cholesterol Education Program – Adult Treatment Plan III, with Asian modification for abdominal circumference. Among all the parameters there was a statistically significant difference only between the percentages of subjects fulfilling the criteria of low HDL among cases and controls.
Comparison between percentages of cases and controls fulfilling each of the criteria of MS.
| Parameter | Cases | Controls | p-value |
|---|
| Number(out of 30) | Percentage(%) | Number(out of 30) | Percentage(%) |
|---|
| FBS> 100 mg/dl | 15 | 50 | 18 | 60 | 0.436 |
| Reduced HDL-c levels | 26 | 86.7 | 18 | 60 | 0.02* |
| elevated serum TG | 12 | 40 | 9 | 30 | 0.417 |
| Elevated BP | 10 | 33 | 10 | 33 | |
| Increased waist circumference | 14 | 46.7 | 18 | 60 | 0.301 |
*Statistically significant (p-value <0.05) as calculated by chi-square test. For the rest of the parameters the difference is not statistically significant as calculated by chi-square test.
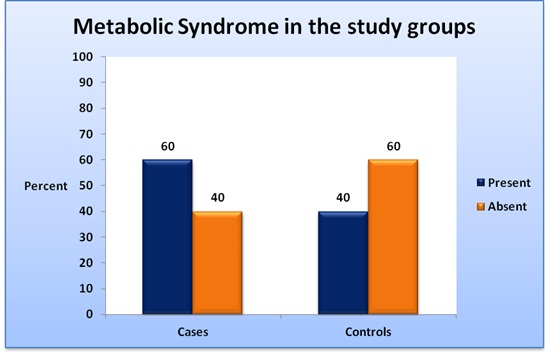
Among the psoriasis cases, 18 (60%) had metabolic syndrome whereas among the control subjects 12 (40%) had the condition [Table/Fig-6]. The difference between the prevalence in the 2 groups was not statistically significant (p-value 0.121). Among 30 psoriasis patients 18 had metabolic syndrome and 12 did not have the condition. The median of duration of psoriasis among patients with metabolic syndrome was 6 years whereas that among psoriasis patients without metabolic syndrome was 5 years. The difference was not statistically significant (p-value 0.656) [Table/Fig-7].
Comparison of prevalence of MS among cases and controls.
The values are for 30 cases and 30 controls. The difference is not statistically significant (p-value > 0.05) as calculated by chi-square test.
Comparison of the duration of psoriasis among psoriasis patients with MS and those without MS.
| Parameter | Psoriasis without MS | Psoriasis with MS | p-value |
|---|
| Median | Range | Median | Range |
|---|
| Duration of disease (years) | 5 | 2.25, 9.5 | 6 | 2, 8.5 | 0.656 |
The comparison is between 18 patients with MS and 12 patients without MS. The difference is not statistically significant (p-value > 0.05) as calculated by Mann-Whitney U test.
Discussion
The link between psoriasis and metabolic syndrome has been explored in a large number of studies of varying methodologies in different parts of the world with a large variation in the findings [15–19]. The few Indian studies conducted in different parts of the country also achieved varied results as mentioned in [Table/Fig-8] [8,20–24].
Metabolic syndrome among psoriasis patients and controls in India.
| Study | Geographicalarea | Metabolicsyndromeamongpsoriasispatients | Metabolicsyndromeamongcontrols | Statisticallysignificant | WhetherAsianmodificationof WCcriteria wasused fordiagnosis ofmetabolic syndrome |
|---|
| Nisa et al., [8] | Srinagar | 28%(42/150) | 6%(9/150) | Yes | No |
| Khunger et al., [20] | New Delhi | 30%(15/50) | 8%(16/50) | Yes | Yes |
| Madanagobalane et al., [21] | Chennai | 44%(52/118) | 30%(36/120) | Yes | Yes |
| Pereira et al., [22] | Mumbai | 18.2%(14/77) | 17.4%(16/92) | No | No |
| Lakshmi et al., [23] | Pondicherry | 32.5%(13/40) | 30%(12/40) | No | No |
| Gopal et al., [24] | Bangalore | 8%(8/100) | 9%(9/100) | No | No |
| Present study | Pondicherry | 60%(18/30) | 40%(12/30) | No | Yes |
The mean age of psoriasis patients in our study was 42.07 years, similar to international studies as well as studies done in India including a previous one done in Pondicherry [17,19,21,23,24]. Thus the average age of our psoriasis group confirmed to the average age of Type 2 psoriasis patients worldwide. A slight male preponderance in our study was similar to studies from the United Kingdom and south India but different from a study done in Turkey where a female preponderance was seen [16,17,21]. Lakshmi et al., from the same geographic area found a marked male preponderance [23]. Most of the international as well as Indian studies failed to show a significant difference in the BMI between cases and controls similar to our study [17,21]. The mean BMI of psoriasis patients in our study was 24.23, lower than most of the studies [16,19,21]. On the other hand, an Indian study done from Srinagar showed a mean BMI among psoriasis patients to be 23.94 similar to our study [8]. The previous study done in Pondicherry showed a slightly lower BMI of 22.47 among psoriasis patients [23]. The percentage of subjects with history of smoking among patients and healthy controls did not differ significantly in our study, similar to the large Indian study done in a population genetically, geographically and socio-economically close to our population [21]. Similarly, the percentage of subjects with a history of alcohol consumption among cases and controls was not significantly different in our study, a finding shared by the above mentioned Indian study [21]. However the percentage of patients with history of alcohol consumption in our study was much higher (50%) compared to the study done by Madanagobalane et al., (21%) [21]. This observation can be explained by the high level of alcoholism in the general population in Puducherry [25].
Analysing the individual components of metabolic syndrome, in our study, the mean waist circumference of psoriasis patients was lesser (85.36 cm) compared to the controls (89.28 cm) but the difference was statistically insignificant. Similarly increased waist circumference – in Asian populations, ≥ 90 cm in men and ≥ 80 cm in women, as a criterion of metabolic syndrome was fulfilled by more individals in the control group (60%) than cases (46.7%). Most of the international studies indicate that obesity is more common among psoriasis patients than controls [15,16,26]. Some of them even show that abdominal obesity as the most common component of metabolic syndrome in psoriasis patients [15,19]. A Turkish study and the previous study done in Pondicherry, however found a lower waist circumference in psoriasis patients compared to controls similar to our study [17,23]. Most of the Indian studies except one from Delhi failed to find a significant independent association between obesity and psoriasis [8,20–22,24]. This indicates that obesity is not an important component of metabolic syndrome in Indian psoriasis patients, a fact corroborated by our study.
Both mean systolic and diastolic blood pressures were lesser among psoriasis patients as compared to controls in our study. Elevated blood pressure systolic ≥130 mm of Hg and/or diastolic ≥ 85 mm of Hg as a criterion of metabolic syndrome was found in equal percentage of cases and controls. This is in disagreement with other studies done in India as well as abroad where systolic and diastolic blood pressures were found to be significantly higher in psoriasis patients compared to controls [8,15,20]. However, some studies failed to show such an association [21–23]. This finding in our study could be explained by the increased blood pressure among control population in our study (33%) similar to another study from south India (27%) done on a similar population [21]. This was much higher than hypertension observed among control population in Delhi, in northern part of India (10%) [20].
The mean fasting serum blood glucose level was higher in controls (134.17 mg/dL) compared to psoriasis patients (118.40) but the difference was statistically insignificant. Elevated blood glucose level ≥ 100 mg/dL as a criterion of metabolic syndrome was found in 60% controls and 50% of cases. This is in disagreement with most of the international studies where raised fasting glucose and diabetes mellitus are more prevalent in psoriasis patients [3,16,17,26,27]. The Indian scenario is also similar [8,21,22,24,28]. A study in Spain however did not show a significantly raised blood glucose level in psoriasis patients in comparison to controls [15]. Our findings are however similar to a study done in Delhi where no significant difference was reported between cases and controls regarding number of subjects with elevated blood glucose and the previous study in Pondicherry where the average fasting blood glucose was similar among psoriasis cases and controls [20,23]. It can be speculated that this unexpected finding in our study resulted from a high prevalence of diabetes mellitus in the general population of Pondicherry aged above 40 years as already reported [29].
In our study, the median for serum triglyceride level for psoriasis patients (145 mg/dL) was higher than controls (120.5 mg/dL), though not statistically significant. Forty percent of cases fulfilled elevated triglycerides ≥150 mg/dl as a criterion of metabolic syndrome, compared to 30% of controls. On the other hand, the mean serum HDL was 37.77 mg/dL among cases, lower than the controls (38.93 mg/dL). As a criterion of metabolic syndrome, reduced HDL-c levels i.e. HDL - < 40 mg/dL in males and < 50 mg/dL in females was fulfilled by 86.7% of cases and 60% of controls, a statistically significant difference. The correlation between dyslipidaemia and psoriasis is variable in different studies. Some of the studies found a significantly higher triglyceride levels in psoriasis patients compared to controls without any significant difference in HDL levels [8,21]. In fact, in both of these studies, a higher number of individuals in the control group fulfilled low HDL as a criterion for metabolic syndrome, compared to psoriasis patients, a finding shared by studies done in Mumbai and Bangalore [22,24]. The previous study from Pondicherry showed a higher average triglyceride level among the patients compared to controls, even though it was not statistically significant. That study also showed paradoxically higher average HDL level among psoriasis patients compared to controls [23]. Our results were similar to studies done in United Kingdom and USA [16,19]. Even though both the mean triglyceride level and prevalence of hypertriglyceridaemia were higher in psoriasis patients compared to controls in our study, the difference did not reach statistical significance probably due to a small sample size. The statistically significant low HDL levels in psoriasis patients could be due to a more sedentary lifestyle and lack of exercise in patients resulting from social stigma and embarrassment. However, since this is a novel finding in the Indian context, it needs to be investigated further by large population-based studies as well as experimental studies to elucidate the molecular aetiopathogenetic links.
Sixty percent of our patients fulfilled the criteria for metabolic syndrome compared to 40% of the control group which however failed to reach statistical significance. This result is in agreement with almost all the studies, which have shown a significantly higher prevalence of metabolic syndrome among psoriasis patients compared to controls [8,16,17,20,21] [Table/Fig-8]. The only study done in the same geographical area with a similar number of patients failed to find a significant difference between frequencies of metabolic syndrome among psoriasis patients and controls [23]. The lack of statistical significance in our study can be explained by our small sample size. Also, we used the same criteria (NCEP ATP-III with Asian modification for abdominal circumference) as the studies done in Chennai and Delhi [20,21]. However, our study may not be comparable with the studies done in Kashmir, Mumbai, Bangalore and Pondicherry as they did not use the Asian modification for waist circumference [8,22,23,24]. This could also be the reason for the much lower frequencies of metabolic syndrome among both psoriasis patients and controls in the previous study done in Pondicherry compared to ours [23].
We failed to find a relation between the presence of metabolic syndrome and duration of psoriasis. This finding was in agreement with most of the studies done in India and abroad [17,18,21,24]. However, our finding was in contrast to the result of the study done in Kashmir, which reported that psoriasis patients with metabolic syndrome had longer mean disease duration than patients without metabolic syndrome [8].
Limitations
However, the study had limitations. This was a hospital based; time bound study with a small sample size, which was the probable reason for the lack of statistical significance, in the analysis of most of the parameters.
Conclusion
Our study linked a lifestyle disorder i.e. metabolic syndrome with a common chronic dermatological condition. It depicted a higher prevalence of metabolic syndrome among psoriasis patients in a south Indian coastal population. However, it did not show significant association of psoriasis with abdominal obesity, hypertension and raised blood glucose underlining the need for larger population-based studies. The positive association of dyslipidaemia with psoriasis indicates the need for routine screening to facilitate early diagnosis and treatment of the condition. The high prevalence of low HDL levels reiterates the need for counselling of patients regarding healthy dietary habits and regular exercise. The high prevalence of raised blood pressure, raised fasting glucose and obesity among the control population too, underlines the need for large-scale, community based programs for health awareness and lifestyle modification.
The values are for 30 cases and 30 controls. The differences are not statistically significant (p-value >0.05) as calculated by student’s t-test
The values are for 30 cases and 30 controls. The differences are not statistically significant (p-value >0.05) as calculated chi-square test.
The values are for 30 cases and 30 controls. The differences are not statistically significant (p-value >0.05) as calculated by student’s t-test.
The values are for 30 cases and 30 controls. The differences are not statistically significant (p-value > 0.05) as calculated by Mann-Whitney U test.
*Statistically significant (p-value <0.05) as calculated by chi-square test. For the rest of the parameters the difference is not statistically significant as calculated by chi-square test.
The comparison is between 18 patients with MS and 12 patients without MS. The difference is not statistically significant (p-value > 0.05) as calculated by Mann-Whitney U test.