Role of progesterone in reproductive medicine is evolving with its suggested clinical role for the hormonal and nonhormonal actions in reproductive medicine. The main function of progesterone is to induce ‘secretory’ changes in endometrium that is further complimented by its immunomodulatory and anti-inflammatory actions. It positively modulates PIBF, NK cells and HOXA 10 genes for better implantation. MHRA recommends Serum Progesterone levels ≥14ng/ml in the mid-luteal phase for supporting pregnancy adequately. Oral Natural Micronized Progesterone SR formulation represents a therapeutic advance in this direction offering ‘therapeutic compliance’ with oral formulation while avoiding the local side effects related to long-term patient compliance in reproductive disorders. The formulation offers round the clock efficiency and efficacy with single dose administration thereby improving patient convenience and compliance. This formulation has been marketed globally since 1986 utilizing the well validated drug delivery system involving Methylcellulose base. The clinical utility of this formulation is further suggested especially in various conditions related with luteal phase insufficiency and Bad obstetric history (BOH) or luteal phase support in ART. The level of evidence has been quite robust with several clinical studies including Prescription Event Monitoring and Investigator initiated studies supporting the clinical role of oral NMP SR formulation especially in ‘Real world’ clinic settings for Luteal phase insufficiency that may be physiological or iatrogenic.
Introduction
Progesterone is an essential hormone which plays an important role in woman’s normal reproductive cycle. The name Progesterone is derived from ‘progestational steroid hormone’ since it helps to prepare and maintain the uterine bed for conception. Progesterone is secreted by ovaries, placenta and adrenal glands and is essential during luteal phase to sustain pregnancy [1]. Also, it promotes shedding of endometrium due to its antiproliferative endometrial action and thereby helps in maintaining a regular menstrual cycle.
Exogenous progesterone supplementation is given for treatment of secondary amenorrhea, dysfunctional uterine bleeding, and endometriosis, for management of luteal phase deficiency, for preventing preterm delivery and also to prevent endometrial hyperplasia [2].
In such cases the role of Natural or Synthetic Progesterone is often highlighted.
Progesterone is available as Natural Progesterone and Synthetic Progestin. The Natural Progesterone is obtained from soybeans and Mexican yam roots [3] and it shows the same chemical structure as that of physiological progesterone found in human body. Micronization of the Natural Progesterone increases the half-life of progesterone with the metabolites including Allopregnanolone showing indirect stimulatory effect on progesterone receptor.
Micronization decreases particle size and enhances the dissolution of progesterone [4]. Absorption of micronized progesterone is enhanced twofold when the hormone is taken with food [5]. Unlike synthetic progestins, micronized progesterone has not been shown to affect mood [6], decrease high-density lipoprotein (HDL) cholesterol levels [7,8] or adversely affect pregnancy outcome [9].
The pharmacologic effects of synthetic progestins (e.g., Norethindrone, Norgestrel, Levonorgestrel, Medroxyprogesterone acetate, Norgestimate) are slightly different from those of natural progesterone. Some of the androgenic effects of synthetic progestins include fluid retention, reduction of HDL cholesterol levels, headaches and mood disturbance.
Dydrogesterone or retroprogesterone is a stereoisomer of progesterone that is a highly selective synthetic progestin binds almost exclusively to progesterone receptor due to its retrostructure [10].
Progesterone Actions: Hormonal Action of Progesterone helps in implantation of fertilized egg in the following ways: i) Stimulates the growth of uterus; ii) Maintains uterine quiescence by stabilizing lysosomal membranes & inhibiting PG synthesis; iii) Inhibits myometrial contractions; iv) Endometrial changes: Induces secretory transformation, increases vascularity of the endometrial lining, stabilizes endometrium [11]. The nonhormonal actions include: i) Immunomodulatory or anti-inflammatory: Progesterone along with HCG and cortisol inhibits the tissue rejection and protect the conceptus. Progesterone blocks the chemokines - transcription factor, NF- kB leading to decreased PG synthesis & release while positively regulating PIBF (Progesterone Induced Blocking Factor), NK (Natural Killer cells), HOX-10, trophoblast HLA gene leading to favorable shift of Th1-Th2 balance towards Th2 type [12].
(Refer [Table/Fig-1]) below; ii) Uterine relaxant: Progesterone blocks the oxytocin effect of prostaglandin F2 α and α-adrenergic stimulation. Similarly it reduces intracellular calcium concentrations and reduces contractility along with lowering the amount of phosphorylated myosin and promote myometrial relaxation.
Nonhormonal actions of Natural progesterone.
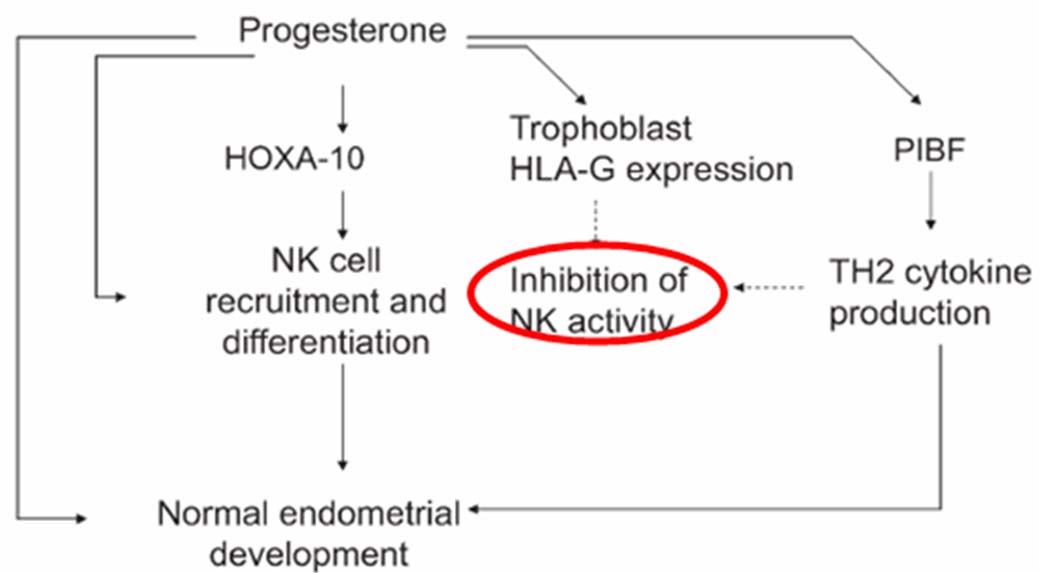
Natural progesterone is often administered through several routes of administration with the local or vaginal therapy often considered to be specific in offering targeted delivery to uterus however the side effects in form of vaginal discharge, pruritus, vaginal messiness and irritation are often important causes for patient noncompliance [13].
Oral Natural Micronized Progesterone SR (Oral NMP SR): There has been a renewed interest and clinical unmet need to derive the benefits of oral administration strategy since it promises compliance and convenience for the patient. The sustained release (SR) tablet formulation currently marketed contains progesterone in a methyl- cellulose base which hydrates in the gastrointestinal tract providing a slow release matrix for a gradual release of progesterone [14].
Oral NMP SR shows slow sustained release pattern over 24 hours while demonstrating long elimination half-life of 18 hours with high protein binding of 90-99% leading to once a dosage convenience. The ‘Smooth’ Release pattern avoids sudden drug release or ‘Dose Dumping’ and therefore loss of drug due to hepatic metabolism [14]. This also minimizes dose related central side effects i.e. drowsiness.
Oral NMP SR has been developed and marketed internationally by Madison Pharmacy® Associates, Madison Wisconsin since 1986.
MHRA (Medicines and Healthcare Products Regulatory Agency) recommends Serum Progesterone levels ≥14ng/ml in the mid-luteal phase for maintaining pregnancy [15].
Oral NMP SR in Obstetrics
A) Bad Obstetric History (BOH) & Luteal phase insufficiency: BOH is one of the common obstetric conditions. BOH is best represented by various clinical states including, Still birth, Preterm or IUGR fetus, Prolonged labour, IUD, Recurrent pregnancy loss. Common causes of BOH include maternal age older than 35 years, multiple pregnancies, structural uterine abnormalities, polycystic ovaries, autoimmune disorders, infections, poorly controlled diabetes, abnormal hormonal response including luteal phase insufficiency and environmental factors [16].
In women with spontaneous or recurrent miscarriage supplementary progesterone provides uterine quiescence avoiding uterine contractions due to inherent lack or deficiency of physiological progesterone [15].
This Luteal-phase insufficiency is primarily a progesterone deficiency defect which is best characterized as a subtle form of ovulatory dysfunction [17]. The inadequate production of progesterone by corpus luteum resulting in insufficient endometrial maturation (for proper placentation) [18] has been proposed to result in LPD. A patient of LPD is subfertile and has following clinical features: advanced age, low body weight, and/or short menstrual cycles which are often accompanied by premenstrual spotting.
Also, it should be noted that the use of ovulation induction methods may compromise the luteal phase. Oral Natural Progesterone is used to improve luteal function while prolonging pregnancy significantly in ‘High risk’ cases including patients with second trimester loss [17].
Natural micronized progesterone offers complimentary immuno-modulatory properties related to positive regulation of PIBF, NK cells and HOXA 10 gene modulation for better endometrial implantation [12]. In line supplementation with progesterone holds greater promise since one of the important predictor for poor outcome in pregnancy has often been correlated with low levels of serum progesterone (ie, <14 ng/mL) [15]. Further, literature review suggests that first trimester Sr. Progesterone level of ≥ 25ng/ml often excludes ectopic pregnancy while a value of ≤ 5ng/ml is predictive for missed abortion [19].
Scientific evidence
In a proof of concept study by Frishman et al., seven women with LPD documented endometrially as ‘out of phase’ biopsies were successfully treated with oral natural micronized progesterone administered as 100 mg tds [20]. At the end of the study, all of the women had their LPD corrected with the serum progesterone achieved systemically corroborating successfully with the ‘in phase’ endometrial biopsies.
In a nationwide PEM study involving 153 patients, conducted to understand the safety profile of oral NMP SR, prescribing doctors considered oral NMP SR as a clinically feasible option for BOH cases with suspected luteal phase insufficiency [Table/Fig-2]. The drug had negligible side effect with dizziness in 0.6%, nausea in 1.3% and giddiness in 0.3% patients [21]. The subgroup analyses showed that Oral NMP SR was administered to women with bad obstetric history showing mean abortion rate as ≤2.
A pilot study by Ghosh et al., in idiopathic recurrent miscarriage was done to assess sub-endometrial blood flow parameters following dydrogesterone (group A: n=51) and micronized vaginal progesterone (group B:n=50) and pregnant women without history of recurrent miscarriage served as controls (group C:n=32) [22]. It was found that after progesterone supplementation, group A and B showed a highly significant reduction in resistivity index (RI), pulsatility index (PI) and an increase in end diastolic velocity (EDV) and pregnancy rates.
Progesterone SR: Supplementation for ‘Suspected’ LPD.
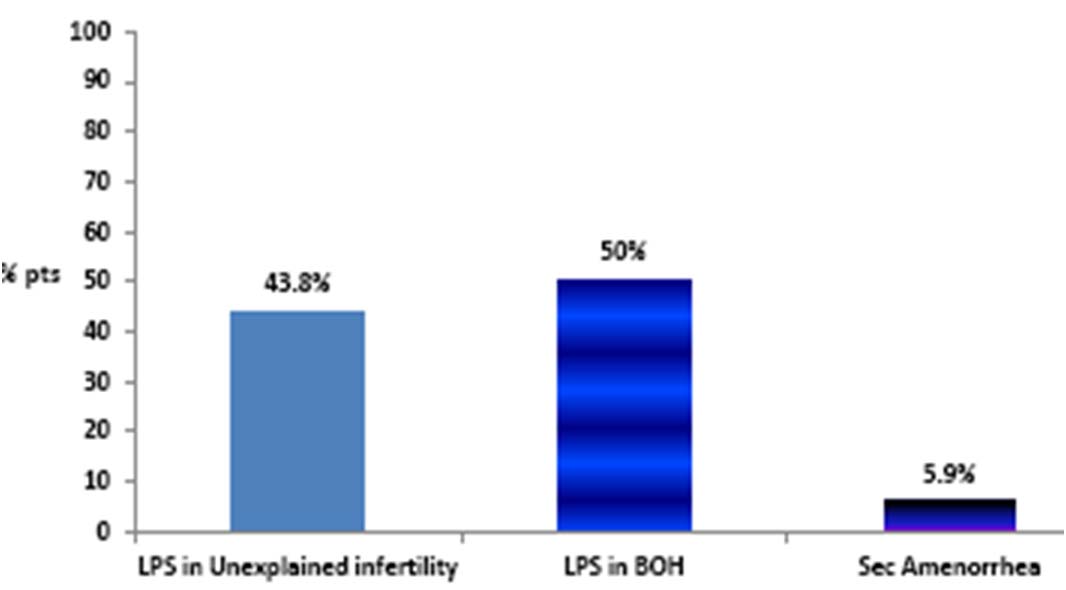
B) Preterm Birth Prophylaxis: According to the recent data available it has been suggested that progesterone is important for uterine quiescence in the latter half of pregnancy. It limits the production of stimulatory prostaglandins and inhibits the expression of contraction- associated protein genes (ion channels, oxytocin and prostaglandin receptors, and gap junctions) within the myometrium [23]. It has also been found that the onset of labour both at term and preterm is associated with a functional withdrawal of progesterone activity at the level of the uterus [23]. This data provides the rationale for the use of oral natural micronized progesterone to prevent preterm labour and birth [24,25]. It has been suggested by the American College of Obstetrics and Gynecology that progesterone use to prevent preterm birth should be restricted to women with prior history of spontaneous preterm delivery at less than 37 weeks or to use in women incidentally (i.e. without routine screening) found to have a short cervix (i.e. less than 15 mm) [26]. Also, progesterone supplementation reduces the risk of low birth weight (<2.5kg) in the newborn.
Scientific evidence
1. In a study conducted on 90 women at 24-34 weeks of singleton pregnancy and arrested preterm labour, Choudhary et al., concluded that maintenance tocolysis with oral micronized progesterone significantly prolonged pregnancy and decreased the number of preterm births [27].
2. Glover et al., [25] documented the clinical utility of oral NMP in patients with history of recurrent spontaneous preterm births.
C) Luteal phase support in ART: Progesterone is used in fertility care with ART. Adequate levels of progesterone are essential to support implantation and early pregnancy. Endogenous levels of progesterone may well be insufficient: at the time of oocyte retrieval, aspiration of follicles may result in the removal of granulosa cells. Therefore, levels of luteal-phase progesterone may not be adequate to support the endometrium and early pregnancy. Also administration of other agents, such as gonadotropin agonists alone or with Clomiphene citrate, also may be associated with diminished or defective progesterone production during the luteal phase. Due to high risk of ovarian hyperstimulation syndrome associated with luteal-phase HCG support, progesterone support is preferable [17].
Scientific Evidence
In a multicentric, randomized, open label, comparative trial conducted by Pouly et al., the clinical utility of natural progesterone administered orally was evaluated in 283 women as Luteal phase support following embryo transfer as part of an IVF procedure [28]. These patients were randomized to receive local progesterone pessary (8% gel) or Natural micronized progesterone capsule as 300 mg/d dose (100 mg in morning & 200 mg at night). Results showed that the pregnancy rates per transfer were not significantly different between the two arms at days 12, 30 or 90. The delivery rates (number of deliveries per patient; 23.0%, vs 22.2%, p = 1.00), as well as the ratio of newborn babies per embryo transferred (11.7% vs. 11.1%, p = 0.91), were also not significantly different between the two groups of Progesterone pessary (8% gel) and NMP capsules respectively.
Gopinath et al., had conducted open label observational study to determine the success rate of first cycle intrauterine insemination (IUI) involving luteal phase support with oral natural or synthetic progesterone [29]. It was found that the therapeutic levels of progesterone achieved with oral NMP SR was almost similar to that of Dydrogesterone for LPS following clomiphene citrate in patients undergoing Natural cycles for unexpained infertility as highlighted below for the first cycle [26] [Table/Fig-3].
Clinical results for the First cycle in patients undergoing Natural cycles for Unexplained infertility.
| Mean Mid-lutealSr. progesterone (ng/ml) | Pregnancy outcome (no.) confirmed by Biochemical assay |
|---|
| Oral NMP SR* | 46.2 | 2 |
| Dydrogesterone | 51.7 | 1 |
(A prospective, randomised study was conducted by Baidya Nath Chakravarty et al., to determine the efficacy, safety and tolerability of vaginal micronized progesterone with oral dydrogesterone as luteal phase support after in-vitro fertilisation (IVF) [30]. A total of 430 women underwent IVF/intracytoplasmic sperm injection (ICSI) treatment. Luteal support was initiated from the day of embryo transfer and continued for up to 14 days. Patients were randomized to luteal supplementation with either intravaginal micronised progesterone 200 mg three times daily (n = 351) or oral dydrogesterone 10 mg twice daily (n = 79). Both dydrogesterone and micronized progesterone were associated with similar rates of successful pregnancies.
Oral NMP SR: Progesterone Assessment Survey
In order to further consolidate the clinical relevance & utility of progesterone supplementation in ‘Real’ world settings, a National Epidemiological Survey using Progesterone Assessment Survey Sheet (PASS) was carried out with responses from 925 Gynecologists across India. The clinicians preferred to use oral NMP SR in various clinical states including LPD, LPS for ART & preterm birth prophylaxis. BOH due to progesterone insufficiency assessed as hormonal assay OR short menstrual cycle history were often treated with vaginal progesterone (58%) or oral NMP SR (36%) as highlighted in [Table/Fig-4].
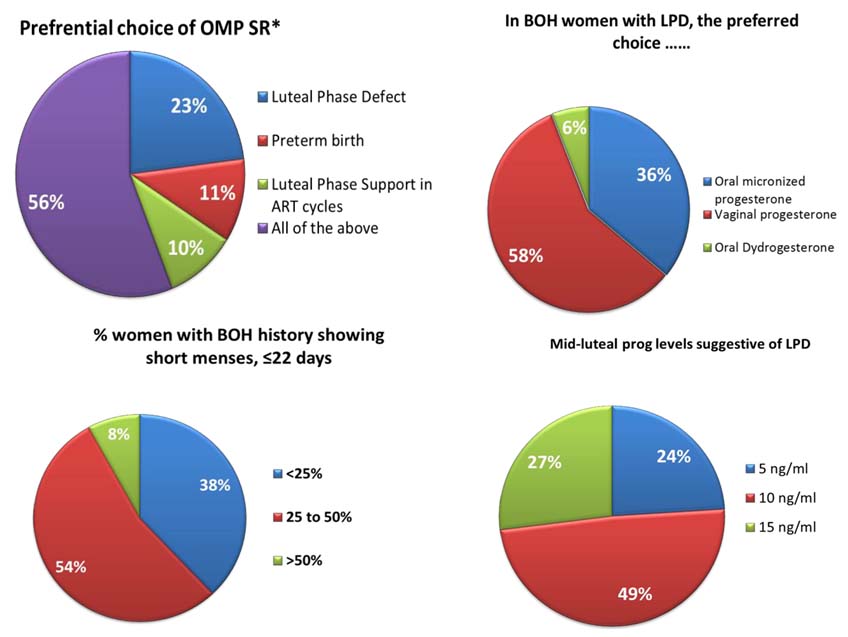
Conclusion
Review of findings from various studies including Epidemiological Survey conducted suggests Natural Progesterone administered orally as Sustained Release (SR) formulation have significant beneficial role in LPD, LPS in ART, BOH & for preterm labour. The Monolith dissolution controlled delivery system of oral NMP SR offers improved patient compliance and convenience due to once a day dosing and clinically feasible option as it achieves Mid-luteal ‘Therapeutic’ levels of Sr. progesterone ≥ 14ng/ml as suggested by MHRA guideline.
[1]. Schindler AE, Differential effects of progestins on hemostasisMaturitas 2003 (suppl 1):S31-S37. [Google Scholar]
[2]. Simon JA, Introduction: An Overview of Progesterone and progestinsThe Journal of Family Practice 2007 (Suppl):3 [Google Scholar]
[3]. Peterson CM, Progestogens, progesterone antagonists, progesterone, and androgens: synthesis, classification, and usesClin Obstet Gynecol 1995 38:813-20. [Google Scholar]
[4]. Barbara S, Apgar, Grant Greenberg, Using Progestins in Clinical PracticeAm Fam Physician 2000 62(8):1839-46. [Google Scholar]
[5]. Simon JA, Robinson DE, Andrews MC, Hildebrand JR, Rocci ML, Blake RE, The absorption of oral micronized progesterone: the effect of food, dose proportionality and comparison with intramuscular progesteroneFertil Steril 1993 60:26-33. [Google Scholar]
[6]. Sherwin BB, The impact of different doses of estrogen and progestin on mood and sexual behavior in postmenopausal womenJ Clin Endocrinol Metab 1991 72:336-43. [Google Scholar]
[7]. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI TrialJAMA 1995 273:199-208. [Google Scholar]
[8]. Ottosson UB, Johansson BG, von Schoultz B, Subfractions of high-density lipoprotein cholesterol during estrogen replacement therapy: a comparison between progestogens and natural progesteroneAm J Obstet Gynecol 1985 151:746-50. [Google Scholar]
[9]. Cornet D, Alvarez S, Antoine JM, Tibi C, Mandelbaum J, Plachot M, Pregnancies following ovum donation in gonadal dysgenesisHum Reprod 1990 5:291-93. [Google Scholar]
[10]. Schindler AE, Classification and Pharmacology of ProgestinsMaturitas The European Menopause Journal 2003 :S7-16. [Google Scholar]
[11]. Chakravorty BN, Endocrinology in relation to reproduction. In: D C Dutta’s (ed.)Textbook of Obstetrics 2011 7th EditionNew Delhi, IndiaJaypee Brothers Medical Publishers (P) Ltd:57 [Google Scholar]
[12]. Szekeres-Bartho J, Balasch J, Progestagen therapy for recurrent miscarriageHuman Reproduction Update 2008 14(1):27-35. [Google Scholar]
[13]. Tomic V, Tomic J, Klaic DZ, Kasum M, Kuna K, Oral dydrogesterone versus vaginal progesterone gel in the luteal phase support: randomized controlled trialEur J Obstet Gynecol Reprod Biol 2015 186:49-53. [Google Scholar]
[14]. Kirk PE, A pharmacokinetic study of micronized natural progesterone extended release tabletsRestore health 1997 [Google Scholar]
[15]. MHRA UK Public Assessment Report. Efficacy of progestogens in the maintenance of early pregnancy in women with threatened miscarriage or recurrent miscarriage. February 2008. Available from: http://www.mhra.gov.uk/home/groups/pl-p/documents/ websiteresources/ con2033921.pdf. [Accessed on 8th July 2015] [Google Scholar]
[16]. Al-Hilli NM, Al-Mosawi HM, The Prevalence of Anticardiolipin Antibodies in women with Bad Obstetric HistoryInt J Curr Microbiol App Sci 2014 3(2):547-53. [Google Scholar]
[17]. Rai P, Rajaram S, Goel N, Gopalkrishnan RA, Agarwal R, Mehta S, Oral Micronized progesterone for prevention of preterm birthInt J Gynecol Obstet 2009 104:40-43. [Google Scholar]
[18]. Ford HB, Schust, DJ, Recurrent pregnancy loss: etiology, diagnosis and therapyReviews in Obstetrics & Gynecology 2009 2(2):76-83. [Google Scholar]
[19]. Stovall TG, Ling FW, Andersen RN, Buster JE, Improved sensitivity and specificity of a single measurement of serum progesterone over serial quantitative beta-human chorionic gonadotrophin in screening for ectopic pregnancyHuman Reproduction 1992 7(5):723-25. [Google Scholar]
[20]. Frishman GN, Klock Sc, Luciano AA, Nulsen JC, Efficacy of Oral Micronized Progesterone in the treatment of Luteal Phase DefectsThe journal of Reproductive Medicine 1995 40(7):521-24. [Google Scholar]
[21]. Purandare AC, Hajare A, Krishnaprasad K, Bhargava A, Prescription Event Monitoring Study To Assess The Safety Profile Of Oral Natural Micronized Progesterone Sustained Release In IndiaInternational Journal of Medical Research & Health Sciences 2014 3(4):975-76. [Google Scholar]
[22]. Ghosh S, Assessment of sub-endometrial blood flow parameters following dydrogesterone and micronized vaginal progesterone administration in women with idiopathic recurrent miscarriage: apiot studyJ Obstet Gynaecol Res 2014 40(7):1871-76. [Google Scholar]
[23]. Norwitz ER, Phaneuf LE, Caughey AB, Progesterone Supplementation and the Prevention of Preterm BirthRev Obstet Gynecol 2011 4(2):60-72. [Google Scholar]
[24]. Keirse MJ, Progestogen administration in pregnancy may prevent preterm deliveryBr J Obstet Gynaecol 1990 97(2):149-54. [Google Scholar]
[25]. Glover MM, A randomized trial of micronized progesterone for the prevention of recurrent preterm birthAm J Perinatol 2011 28(5):377-81. [Google Scholar]
[26]. Use of progesterone to reduce preterm birth. ACOG Committee Opinion No. 419. American College of Obstetricians and GynecologistsObstet Gynecol 2008 112:963-65. [Google Scholar]
[27]. Choudhary M, Suneja A, Vaid N, Guleria K, Faridi MMA, Maintenance tocolysis with oral micronized progesterone for prevention of preterm birth after arrested preterm labourInt J Gynecol Obstet 2014 126(1):60-63. [Google Scholar]
[28]. Pouly JL, Bassil S, Frydman R, Hedon B, Nicollet B, Prada Y, Luteal support after in-vitro fertilization: Crinone 8%, a sustained release vaginal progesterone gel, versus Utrogestan, an oral micronized progesteroneHuman Reproduction 1996 11(10):2085-89. [Google Scholar]
[29]. Gopinath PM, Desai RR, Open Label Observational Study To Determine The Success Rate of First Cycle Intrauterine Insemination(IUI) Involving Luteal Phase Support With Oral Natural Or Synthetic ProgesteronInternational Journal of Medical Research & Health Sciences 2014 3(4):933-36. [Google Scholar]
[30]. Chakravarty BN, Shirazee HH, Dam P, Goswami SK, Chatterjee R, Ghosh S, Oral dydrogesterone versus intravaginal micronised progesterone as luteal phase support in assisted reproductive technology (ART) cycles: Results of a randomised studyThe Journal of Steroid Biochemistry and Molecular Biology 2005 97(5):416-20. [Google Scholar]