Introduction
Oxidative stress or oxidant/antioxidant imbalance has a crucial role in the pathogenesis of some diseases like cancer. Medullary thyroid carcinoma (MTC) originates in the thyroid parafollicular cells and includes 3-4% of the malignant neoplasms that have an effect on this gland. The aetiology of MTC has not been clarified. However, oxidative stress may be one of the factors involved.
Aim
The aim of the current study was to evaluate the antioxidant enzyme activity of catalase (CAT), Glutathione (GSH), total antioxidant capacity (TAC) and the levels of the lipid peroxidation product malondialdehyde (MDA) in blood samples of MTC patients as compared to healthy controls.
Materials and Methods
A case-control study was designed enrolling patients with confirmed MTC diagnosis and age-and sex group matched healthy volunteers referred to the clinic of the Research Institute for Endocrine Sciences, Tehran, Iran from April 2013 to July 2015. Fasting blood samples were taken for study. Catalase, GSH, MDA and TAC levels were measured by colorimetry using commercial kits (ZellBio GmbH, Germany). Data were analysed using SPSS 17 software, comparing mean±SD through t-test and difference between proportions through chi-square.
Results
No statistical difference was observed in the demographic characteristic between cases and controls. The final MTC group included 40 males and 45 females with a mean age of 30±12.9 year, and the control group 40 males and 47 females, with a mean age of 31.2±12.3 year. Anthropometric parameters, dietary and thyroid hormones levels (T3, T4 and TSH) were similar. Serum TAC (p=0.015), GSH (p=0.029) and CAT (p<0.001) levels were found to be significantly lower in the MTC patients, while serum MDA levels were significantly higher in MTC patients than controls (p<0.001).
Conclusion
These preliminary findings suggest that oxidant/antioxidant imbalance may be associated with or possibly indicate an increased risk to medullary thyroid carcinoma. Further studies are needed to explore these findings.
Introduction
Thyroid cancer has the highest prevalence among endocrine malignancy and its incidence continues to rise annually. The main cause for this cancer is not clear, but various risk factors may include exposure to UV radiation, family history, age and low or high amounts of iodine in the diet can increase the risk of the thyroid cancer. According to epidemiological studies, the rate of this malignancy in women is three times more than men, which can be associated with the development and proliferation of estrogen hormone receptors in the process of cancer [1].
Medullary thyroid carcinoma (MTC) which is driven from the thyroid parafollicular or C-cells is responsible for almost 10% of all thyroid cancers [2,3]. MTC is sporadic in nature, however 20-30% of cases have a hereditary pattern, as an autosomal dominant trait. This hereditary pattern is referred to as ‘multiple endocrine neoplasia type 2’ (MEN 2), which is identified by the MTC in addition to pheochromocytoma and hyperparathyroidism (MEN 2A), or MTC in addition to multiple mucosal neuromas, pheochromocytoma, marfanoid habitus and eye abnormalities (e.g., conjunctivitis sicca, thickened corneal nerves, and an inability to cry tears) (MEN 2B) [4,5].
Recently the role of oxidative stress in the pathogenesis of many cancers and their complications has been considered. Oxidative stress is a condition in which the equilibrium between free radical production and their scavenging by antioxidants becomes perturbed and leads to accumulation of free radicals or their products within cells. This imbalance leads to damage of important biomolecules and organs with potential impact on the whole cells. Free radicals are produced from normal metabolic reactions after several steps and its production is inevitable. The main sources of free radicals are microsomal-membrane and mitochondrial electron transfer chains and also auto oxidation reactions [6,7]. Hydroxyl radical can initiate lipid peroxidation and produce MDA, which leads to damage of membrane structure and function [8]. Oxidation reactions can produce free radicals to launch chain reactions that damage cells. The anti-oxidation in the defensive system of the organism terminates these reactions by eliminating of free radical mediators, and inhibits oxidation reactions by their auto oxidiation. The defensive system consists of enzymatic reaction and non-enzymatic reaction. In enzymatic reaction, active sites of many enzymes such as SOD, GSH-Px, CAT and GST are composed of microelements. On the other hand non-enzymatic reaction system is mainly composed of vitamins, amino acids and metal binding proteins, such as VitE, Carotene, VitC, Cys, His, Ser, Met, Glucose, Ceruloplasmin, Transderrin and lactofein [6,7,9].
Upregulation of a wide range of antioxidant genes is one of the main mechanisms by which cells protect themselves against oxidative stress. Among the antioxidants, glutathione (GSH) is the most abundant intracellular non-protein thiol in cells and it functions in the removal of potentially toxic electrophiles and metals, thereby protecting cells from toxic oxygen products [9,10]. Catalase antioxidant enzyme is associated with the anti-oxidation defense. Considering that their effect can be additive, the best approach is to measure the combined activity of all antioxidants or the total antioxidant capacity (TAC) rather than measuring individual activity of different molecules [11].
There is an increasing interest in understanding the potential function of oxidative stress biomarkers including Catalase [12], SOD [13], GPx [12], GSH [14], vitamin E [15], vitamin C [16] in thyroid carcinogenesis. Also, Blood concentrations of glutathione peroxidase (an antioxidant enzyme) and malondialdehyde (a product of lipid peroxidation), were assessed before and after thyroidectomy. The plasma levels of both markers of oxidative stress were significantly higher in patients with thyroid cancer in comparison to the control subjects [17].
To date, our understanding of the roles of the oxidative/antioxidative systems in the carcinogenesis of MTC is limited. In this study we evaluated the levels of antioxidant enzyme activity of Catalase (CAT), and the serum levels of lipid peroxidation product malondialdehyde (MDA), Glutathione (GSH) and total antioxidant capacity (TAC) in MTC patients compared with healthy controls.
Materials and Methods
The study was designed as age- and sex- matched case-control study, conducted at the Research Institute for Endocrine Sciences of Shahid Beheshti University of Medical Sciences in collaboration with Department of Clinical Biochemistry of Hamadan University of Medical Sciences, Iran, during 2013-2015.
Ethical consideration: The study followed the principles of the declaration of Helsinki and was approved by the Medical Ethics Review Board of Hamadan University of Medical Sciences. Written informed consent was obtained from the volunteers regarding confidentiality and anonymity of data collected, also details and purpose of the study were disclosed.
Subjects: From April 2013 to July 2015, 85 patients (12-59-year-old), with confirmed diagnosis of medullary thyroid carcinoma (40 males and 45 females), referred to the Research Institute for Endocrine Sciences, were enrolled in the MTC group. All patients were newly diagnosed as having MTC based on clinical-pathological examination, but with thyroid profile (T3, T4, TSH) within normal range.
A control group was formed by age- and sex-group matched healthy volunteers (40 males and 47 females; 11–58 years old) from other people (not relative) attending the Institute with no history of thyroid disease. Potential participants in both groups were clinically examined and excluded in case of history of serious chronic illnesses of heart, lung, and kidney; prior treatment with thyroid cancer medications for either MTC or conditions associated with thyroid carcinoma; intake of prescribed or over-the-counter any kind of antioxidants (such as vitamins and carotenes) in the past year; history of current or past smoking and lack of informed consent and/or authorization form to the processing of personal data. Finally, demographic data collection form was completed for each of the participants in the study.
Blood samples: Blood samples (3 ml) were collected after 8 hours overnight fasting, from subjects in the antecubital vein of the left arm. After 5 minutes coagulation, the samples were centrifuged 3000 rpm for 10 min and the obtained sera were stored at -80°C until use for the analysis of oxidative stress markers.
Total antioxidant capacity (TAC) Assay: Serum TAC was measured using a commercial kit following the manufacturer’s protocol (ZellBio GmbH, Germany) on the basis of the oxidation reduction colorimetric assay at a wavelength of 490 nm. TAC level was considered as the amount of antioxidant in the sample that was compared with ascorbic acid action as a standard. This method can determine TAC with 0.1 mM sensitivity (100 μmol/L). The intra- and inter-assay variation co-efficient is claimed to be a less than 3.4% and 4.2%, respectively.
Catalase activity (CAT) Assay: CAT activity was measured using a calorimetrically enzymatic assay kit at 405 nm (ZellBio GmbH, Ulm, Germany). In this assay, the CAT activity unit was considered as the amount of the sample that will catalyze decomposition of 1 μmole of H2O2 to water and O2 in one minute. This method can determine CAT with 0.5 U/mL sensitivity. The intra- and inter-assay coefficient of variation was claimed to be 6.3% and 7.9%, respectively.
Malondialdehyde (MDA) Assay: Measurement of MDA level was done by a commercial chemical colorimetrical assay kit according to manufacturer’s protocol (MDA assay kit; ZellBio GmbH, Ulm, Germany). It uses the MDA-TBA adduct formed by the reaction of MDA and thiobarbituric acid (TBA) under high temperature. MDA is measured in acidic media and heat (90-100 °C) colorimetrically at 535 nm. This method can determine the MDA with 0.1 μM sensitivity. The intra- and inter-assay coefficient of variation is claimed to be 5.8% and 7.6%, respectively.
Glutathione (GSH) Assay: Measurement of serum GSH level was done with commercial chemical colorimetric assay kits (ZellBio GmbH, Ulm, Germany) using colorimetrically method at 412 nm. The assay sensitivity is 0.1 mM and intra- and inter-assay coefficient of variation 6.1% and 7.7%, respectively.
Statistical Analysis
Statistical analyses were done by using the program SSPS version 17.0 (SPSS, Inc., Chicago, IL, USA). A chi-square test was used to compare qualitative variables between the two groups. Because all quantitative parameters according to the Kolmogorov-Smirnov test had normal distributions, independent t-test was used for comparison of continuous parameters between the two groups, respectively. The results are expressed as a mean± standard deviation (SD), and/or range (minimum–maximum). p-values less than 0.05 were considered statistically significant.
Results
Cases and controls did not differ with regard to age, sex distribution and body mass index [Table/Fig-1]. Women comprised the majority of subjects in both case and control groups (52.9% and 54% respectively). Anthropometric and dietary habits were similar between the two groups. The thyroid hormones (T3, T4, TSH) levels were within normal limits in all subjects in both groups (data were not shown).
Demographic data of the study participants.
| Cases (N=85) | Controls (N=87) | p-value |
|---|
| Age (yr), Mean±SD | 30±12.9 | 31.2±12.3 | 0.12 |
| Sex (female/male) | 45/40 | 47/40 | 0.48 |
| BMI (kg/m2), Mean±SD | 27±4.9 | 28.2±5.3 | 0.72 |
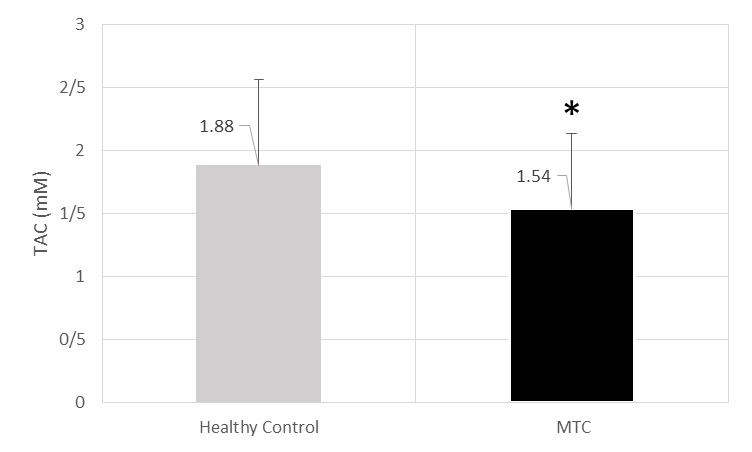
TAC activity was estimated to be 1.54±0.59 mM for cases and 1.88±0.68 U/ml for controls (p=0.015) [Table/Fig-2].
The level of TAC (mM) of MTC and healthy control’s sera.* significant decrease in comparison from healthy control (p=0.015).
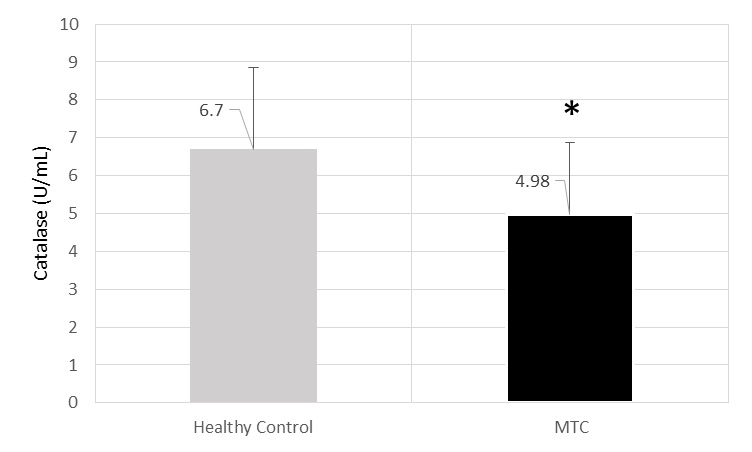
CAT mean activity was 4.98±1.9U/ml for cases and 6.7±2.16 U/ml for controls (p<0.001) [Table/Fig-3].
The level of Catalase (U/mL) of MTC and healthy control’s sera.* significant decrease in comparison from healthy control (p<0.001).
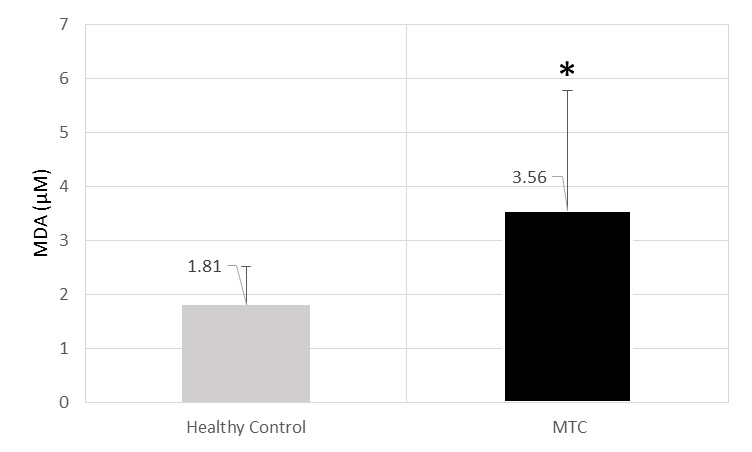
MDA mean level was 3.56±2.21 uM in cases and 1.81±0.71uM in controls (p<0.001) [Table/Fig-4].
The level of MDA (μM) of MTC and healthy control’s sera.* significant increase in comparison from healthy control (p<0.001).
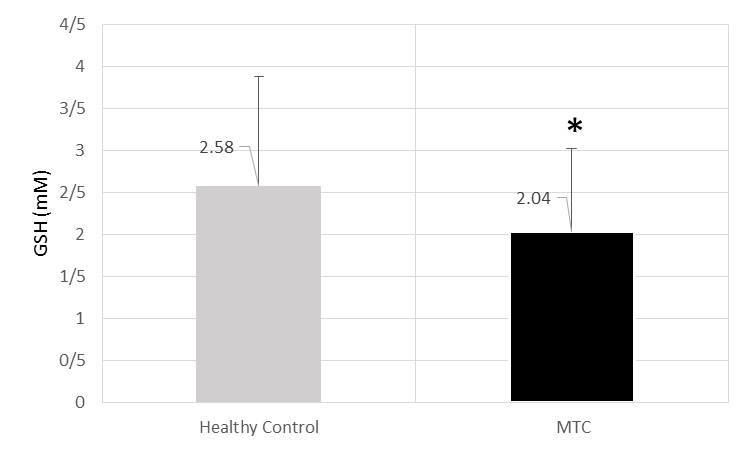
GSH mean activity was 2.04±0.98 mM in cases and 2.58±1.3 mM (p=0.029) [Table/Fig-5].
The level of GSH (mM) of MTC and healthy control’s sera.* significant decrease in comparison from healthy control (p=0.029).
Discussion
In previous investigations, increased oxidative stress in thyroid cancer has been revealed [12,13,15,17]. Our findings showed that MDA levels were increased, while serum TAC, GSH and CAT levels were decreased in MTC patients. These preliminary findings indicate an oxidant/antioxidant imbalance associated with MTC. Free radicals and ROS participate in physiological and pathological process in the thyroid gland. In the recent years, many evidences demonstrated that the free radicals have role in the mechanisms of initiation, development of neoplastic transformations invitro and invivo, also in the stimulation of specific oncogenes. Most of them originate from the fact that the agents that eliminate the free radical or disrupt the chain of events induced by free radical can inhibit the neoplastic process both at the cellular and molecular level [18,19]. Free radicals have influence on the cell components such as DNA, protein, lipid and carbohydrates, and especially lipids are the most sensitive component.
MDA is the end product of lipid peroxidation. Serum MDA level, as a marker for lipid peroxidation, can indicate oxidative damage in cells and tissues. Sadani et al., and Mano et al., have shown an increased level of MDA concentration in papillary carcinoma as compared to those in the normal thyroid tissue, a finding consistence with our observations [18,20]. The balance between the production and elimination of lipid peroxides determines peroxide level in cells. The decreasing of cellular defenses or significant increase in peroxidative reactions can lead to disruption of this balance. The increased lipid peroxidation and the damage of antioxidant defense system may lead to the rising of free radicals in thyroid cancer condition [21]. In addition, oxidation of polyunsaturated fatty acids by ROS leads to the formation of lipid peroxidation products such as MDA [22].
In the present study, GSH level was significantly lower in the MTC patients than the control group. Reduced glutathione, an important intracellular antioxidant, acts both as a co-factor for glutathione peroxidase and as a direct active scavenger to remove reactive species such as hydroxyl radical, carbon centered radicals, peroxynitrite, and singlet oxygen. Reduced glutathione also play a very crucial role in the protection of erythrocyte from oxidative damage [23]. In previous survey, lower levels of reduced glutathione in plasma and erythrocytes have been reported to play a role in various pathological conditions including cancer [24]. GSH has the function to maintain cellular redox status. An increase of GSH levels is likely to reduce ROS levels and antagonize oxidative stress [25]. Long-term oxidative stress can be associated with cell damage because it exceeds the capacity of antioxidant synthesis by the target organs or extracellular [26].
To investigate the antioxidative system in MTC patients, we evaluated serum levels of TAC. The result of our study showed that TAC in MTC patients is significantly less than healthy controls. This is probably due to increased metabolism in result of increased production of free radicals. In this study, CAT activity in MTC patients revealed a significant decrease compared to the control group. Hydrogen peroxide is a normal metabolite in living cells. Catalase (hydrogen peroxide: hydrogen peroxide oxidoreductase, EC 1.11.1.6), known as a defense to oxidative stress, is an anti-oxidant enzyme which catalysis the breaks down of hydrogen peroxide (H2O2) into oxygen and water without producing free radicals. The excess amount of hydrogen peroxide can oxidize cellular components so its removal is vital for cells. All aerobic cytochrome-containing mammalian cells, particularly liver and kidney have some catalase activity. The lowest activity, however, is found in connective tissue [27]. Lipids peroxidation is increased in thyroid cancer malignant tissues while antioxidant scavenging enzymes are reduced, leaving these tissues more susceptible to the toxic effects of free radical. It has been demonstrated that the antioxidative defense can reduce levels of free radicals produced by thyroid gland dysfunction [13].
In fact, serum enzyme activities like CAT or MDA, TAC and GSH levels are good indicators for the systemic oxidant/antioxidant status but do not necessarily indicate their real changes occurring in the thyroid directly, because numerous factors are involved and can modify the serum outcome. The oxidative metabolism can be regulated by the thyroid hormone influencing antioxidants levels such as SOD, GPx, catalase, and glutathione reductase and non-enzymatic antioxidants such as vitamin E and C, glutathione, and uric acid [28]. However, all participants, both in the case and control groups presented a normal thyroid hormone profile.
Limitation
The assessment of more biomarkers in oxidative stress including SOD, GSH-Px, GST, Vit E, Carotene, Vit C, Cys, His, Ser, Met, Glucose, amino acids, Ceruloplasmin, Transderrin and lactofein and also in more bigger population is more helpful to clarified oxidative stress status in patients with MTC which can be mention as a limitation in our study.
Conclusion
Decreased antioxidant GSH, TAC and CAT activity with increased lipid peroxidation product MDA indicate that in thyroid cancer antioxidants’ enzyme activity is decreased and lipid peroxidation products are elevated. These preliminary findings suggest that oxidant/antioxidant imbalance may be associated with or possibly indicate an increased risk to medullary thyroid carcinoma. The significantly increased values of MDA, supports the hypothesis that some disturbances in the oxidant-antioxidant balance in favour of oxidation may have a role in MTC. Further studies are need to explore to clarify the role of oxidative stress in the thyroid carcinogenesis.
[1]. Nozhat Z, Hedayati M, Azizi F, Thyroid Cancer Epidemic: A Peril or an Alarm?International journal of endocrinology and metabolism 2015 13(4):e28491 [Google Scholar]
[2]. Hedayati M, Yeganeh MZ, Sheikholeslami S, Afsari F, Diversity of Mutations in the RET proto-oncogene and its Oncogenic Mechanism in Medullary Thyroid CancerCritical reviews in clinical laboratory sciences 2015 :1-35. [Google Scholar]
[3]. Yeganeh MZ, Sheikholeslami S, Hedayati M, RET proto oncogene mutation detection and medullary thyroid carcinoma preventionAsian Pacific journal of cancer prevention: APJCP 2015 16(6):2107-17. [Google Scholar]
[4]. Nozhat Z, Hedayati M, PI3K/AKT Pathway and Its Mediators in Thyroid CarcinomasMolecular diagnosis & therapy 2015 [Google Scholar]
[5]. Jabbari S, Hedayati M, Yaghmaei P, Parivar K, Medullary Thyroid Carcinoma—Circulating Status of Vaspin and Retinol Binding Protein-4 in Iranian PatientsAsian Pacific journal of cancer prevention: APJCP 2015 16(15):6507-12. [Google Scholar]
[6]. Ha HC, Thiagalingam A, Nelkin BD, Casero RA, Reactive oxygen species are critical for the growth and differentiation of medullary thyroid carcinoma cellsClinical cancer research 2000 6(9):3783-87. [Google Scholar]
[7]. Mates JM, Perez-Gomez C, Nunez de Castro I, Antioxidant enzymes and human diseasesClinical biochemistry 1999 32(8):595-603. [Google Scholar]
[8]. Halliwell B, Gutteridge JM, Role of free radicals and catalytic metal ions in human disease: an overviewMethods in enzymology 1990 186:1-85. [Google Scholar]
[9]. Halliwell B, Oxidative stress and cancer: have we moved forward?Biochem j 2007 401:1-11. [Google Scholar]
[10]. Anderson ME, Glutathione: an overview of biosynthesis and modulationChemico-biological interactions 1998 :111-112.:1-14. [Google Scholar]
[11]. Erel O, A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cationClinical biochemistry 2004 37(4):277-85. [Google Scholar]
[12]. Lassoued S, Mseddi M, Mnif F, Abid M, Guermazi F, Masmoudi H, A comparative study of the oxidative profile in Graves’ disease, Hashimoto’s thyroiditis, and papillary thyroid cancerBiological trace element research 2010 138(1-3):107-15. [Google Scholar]
[13]. Erdamar H, Çimen B, Gülcemal H, Saraymen R, Yerer B, Demirci H, Increased lipid peroxidation and impaired enzymatic antioxidant defense mechanism in thyroid tissue with multinodular goiter and papillary carcinomaClinical biochemistry 2010 43(7):650-54. [Google Scholar]
[14]. Du Z-X, Zhang H-Y, Meng X, Guan Y, Wang H-Q, Role of oxidative stress and intracellular glutathione in the sensitivity to apoptosis induced by proteasome inhibitor in thyroid cancer cellsBMC cancer 2009 9(1):56 [Google Scholar]
[15]. Alkhenizan A, Hafez K, The role of vitamin E in the prevention of cancer: a meta-analysis of randomized controlled trialsAnnals of Saudi medicine 2007 27(6):409 [Google Scholar]
[16]. Liu B, Kuang A, Huang R, Zhao Z, Zeng Y, Wang J, Influence of vitamin C on salivary absorbed dose of 131I in thyroid cancer patients: a prospective, randomized, single-blind, controlled trialJournal of Nuclear Medicine 2010 51(4):618-23. [Google Scholar]
[17]. Akinci M, Kosova F, Çetin B, Sepici A, Altan N, Aslan S, Oxidant/antioxidant balance in patients with thyroid cancerActa Cirúrgica Brasileira 2008 23(6):551-54. [Google Scholar]
[18]. Sadani GR, Nadkarni GD, Role of tissue antioxidant defence in thyroid cancersCancer letters 1996 109(1-2):231-35. [Google Scholar]
[19]. Dumitrescu C, Belgun M, Olinescu R, Lianu L, Bartoc C, Effect of vitamin C administration on the ratio between the pro- and antioxidative factorsRomanian journal of endocrinology / sponsore [sic] by the Academy of Medical Sciences 1993 31(1-2):81-84. [Google Scholar]
[20]. Mano T, Shinohara R, Iwase K, Kotake M, Hamada M, Uchimuro K, Changes in free radical scavengers and lipid peroxide in thyroid glands of various thyroid disordersHormone and metabolic research 1997 29(7):351-54. [Google Scholar]
[21]. Yanagawa T, Ishikawa T, Ishii T, Tabuchi K, Iwasa S, Bannai S, Peroxiredoxin I expression in human thyroid tumorsCancer letters 1999 145(1-2):127-32. [Google Scholar]
[22]. Baz K, Cimen MY, Kokturk A, Yazici AC, Eskandari G, Ikizoglu G, Oxidant / antioxidant status in patients with psoriasisYonsei medical journal 2003 44(6):987-90. [Google Scholar]
[23]. Banerjee KK, Marimuthu P, Sarkar A, Chaudhuri RN, Influence of cigarette smoking on Vitamin C, glutathione and lipid peroxidation statusIndian journal of public health 1998 42(1):20-23. [Google Scholar]
[24]. Gerard-Monnier D, Chaudiere J, Metabolism and antioxidant function of glutathionePathologie-biologie 1996 44(1):77-85. [Google Scholar]
[25]. Dalton TP, Chen Y, Schneider SN, Nebert DW, Shertzer HG, Genetically altered mice to evaluate glutathione homeostasis in health and diseaseFree radical biology & medicine 2004 37(10):1511-26. [Google Scholar]
[26]. Komosinska-Vassev K, Olczyk K, Kucharz EJ, Marcisz C, Winsz-Szczotka K, Kotulska A, Free radical activity and antioxidant defense mechanisms in patients with hyperthyroidism due to Graves’ disease during therapyClinica chimica acta; international journal of clinical chemistry 2000 300(1-2):107-17. [Google Scholar]
[27]. Bai J, Rodriguez AM, Melendez JA, Cederbaum AI, Overexpression of catalase in cytosolic or mitochondrial compartment protects HepG2 cells against oxidative injuryJournal of Biological Chemistry 1999 274(37):26217-24. [Google Scholar]
[28]. Wang D, Feng J-F, Zeng P, Yang Y-H, Luo J, Yang Y-W, Total oxidant/antioxidant status in sera of patients with thyroid cancersEndocrine-related cancer 2011 18(6):773-82. [Google Scholar]