Primary Axillary Porocarcinoma: A Rare Cutaneous Tumour
Nalli R. Sumitra Devi1, K. Valarmathi2, Mary Lilly3, Selvi Satish4, Nidhi Mishra5
1 Professor, Department of Pathology, Govt Stanley Medical College, Chennai, Tamil Nadu Dr. M.G.R. Medical University, India.
2 Professor, Department of Pathology, Govt Stanley Medical College, Chennai, Tamil Nadu Dr. M.G.R. Medical University, India.
3 Professor, Department of Pathology, Govt Stanley Medical College, Chennai, Tamil Nadu Dr. M.G.R. Medical University, India.
4 Assistant Professor, Department of Pathology, Govt Stanley Medical College, Chennai, Tamil Nadu Dr. M.G.R. Medical University, India.
5 Post Graduate, Department of Pathology, Govt Stanley Medical College, Chennai, Tamil Nadu Dr. M.G.R. Medical University, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Nalli R. Sumitra Devi, Professor, Department of Pathology, Govt Stanley Medical College, Chennai, Tamil Nadu Dr.M.G.R. Medical University, India.
E-mail: drnallisumitra@yahoo.co.in
Eccrine porocarcinoma, a rare cutaneous malignant tumour accounts for a fraction of sweat gland tumours. This tumour is found to originate from the intraepithelial parts of the sweat glands. It commonly involves the lower extremities in elderly patients and carries an aggressive behaviour. Cutaneous and visceral metastasis can occur and hence prompt treatment is mandatory. Surgical excision is the mainstay of treatment modality. We hereby present a case of eccrine porocarcinoma in a 50-year-old male in the right axillary region presenting as a verrucous lesion.
Axilla, Eccrine, Malignant, Verrucous lesion
Case Report
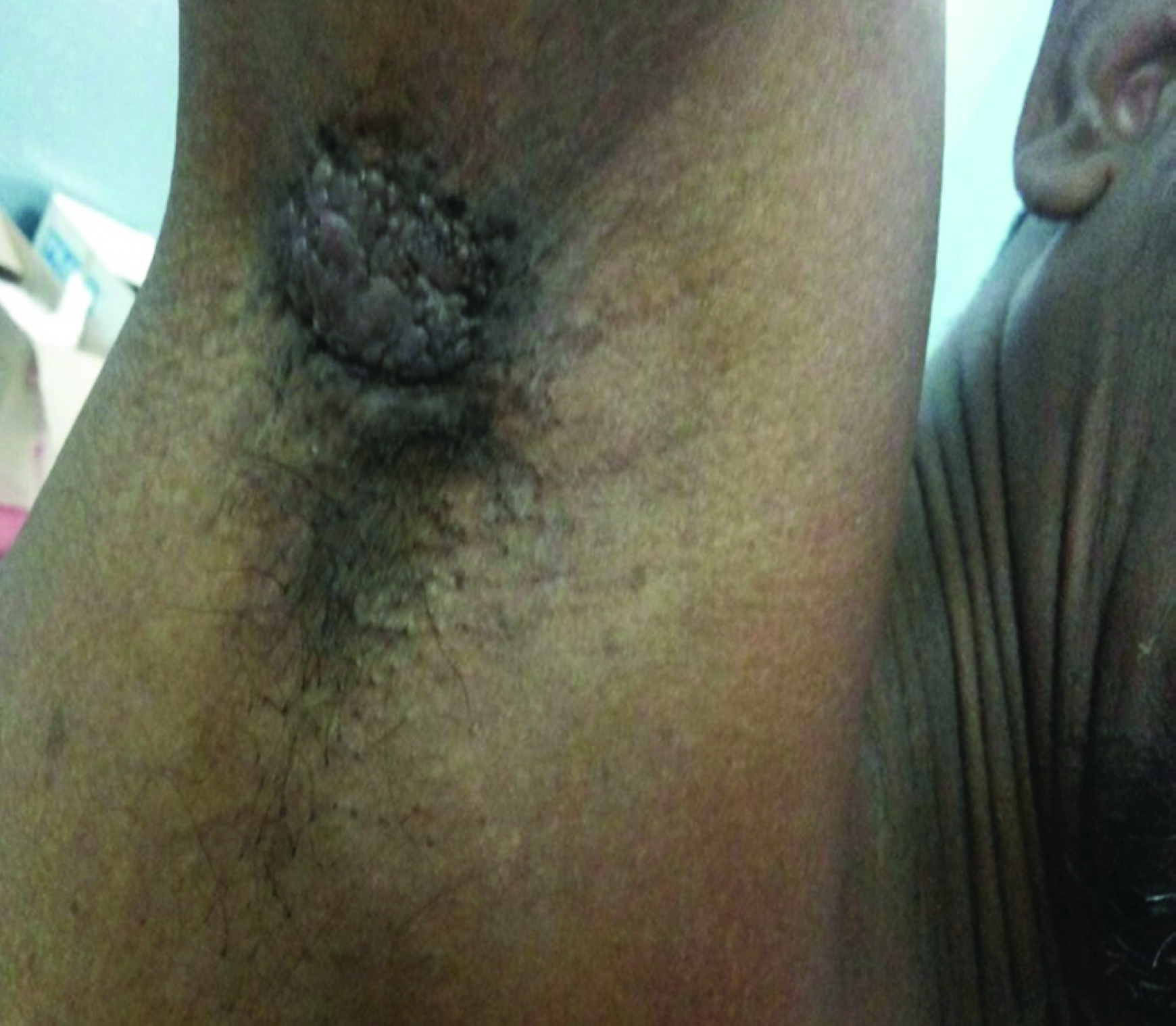
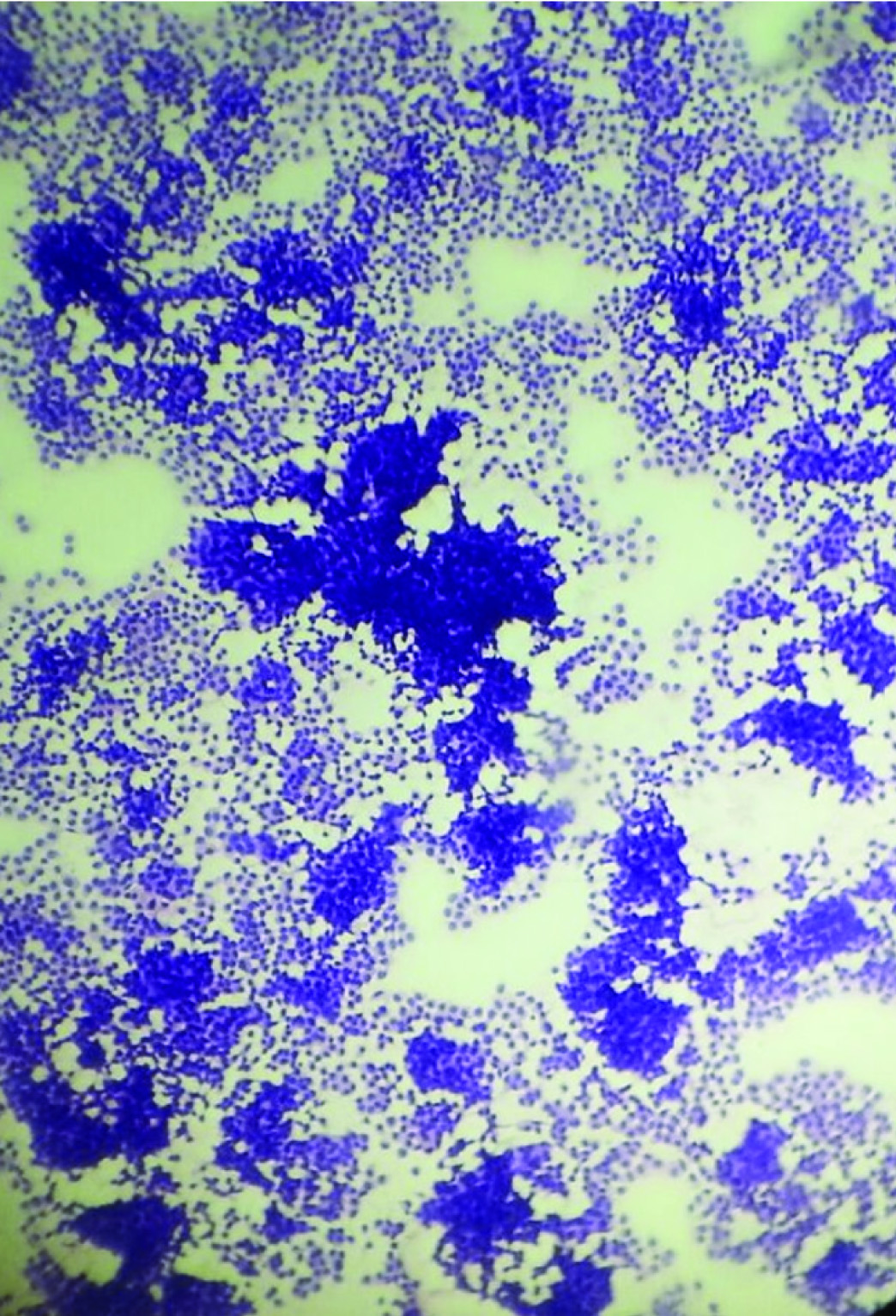
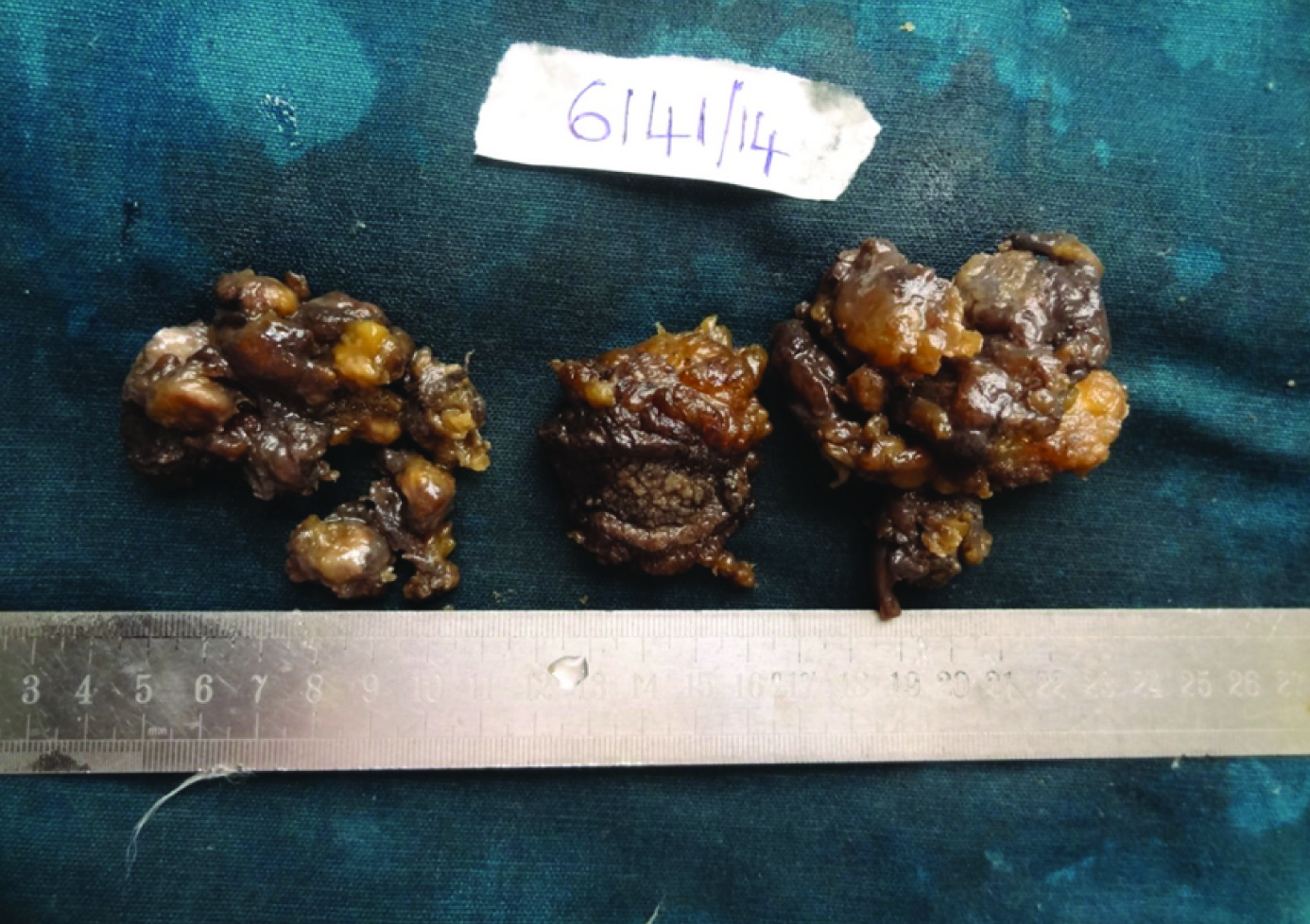
A 50-year-old male presented with a verrucous lesion of 5x4 cm in the right axillary region since 4 years [Table/Fig-1]. Both breasts were clinically normal. General examination revealed 11 lymph nodes, largest measuring 3x2 cm and smallest measuring 1x1 cm. There was no previous history of radiation injury, solar exposure or immunosuppression. There was no history of any systemic disorders. The clinical diagnosis of squamous cell carcinoma was rendered. FNAC showed a cellular smear with discohesive clusters, sheets and singly scattered neoplastic cells having minimal to moderate amount of cytoplasm and hyperchromatic nuclei with some showing prominent nucleoli [Table/Fig-2]. A differential diagnosis of malignant adnexal tumour/ductal carcinomatous deposit in the axilla was offered. Patient was explained about the need for excision for a definite diagnosis. Wide excision was done after obtaining consent from the patient. Gross examination showed a verrucous and multinodular lesion of 4 x 3.5 x 2.5 cm with circumferential margin of 0.5 cm which was grayish white on cut section [Table/Fig-3]. Axillary pad of fat measured 6x3x2 cm and cut section showed 11 lymph nodes, largest measuring 3x2x1 cm and smallest measuring 1x1x0.5 cm which was grayish white on cut section.
5 X 4 cm verrucous lesion- right axilla
FNAC- Malignant cells in discohesive clusters, sheets and singles (H&E, 400X)
Gross- 4 x 3.5 x 2.5 cm verrucous and multinodular lesion
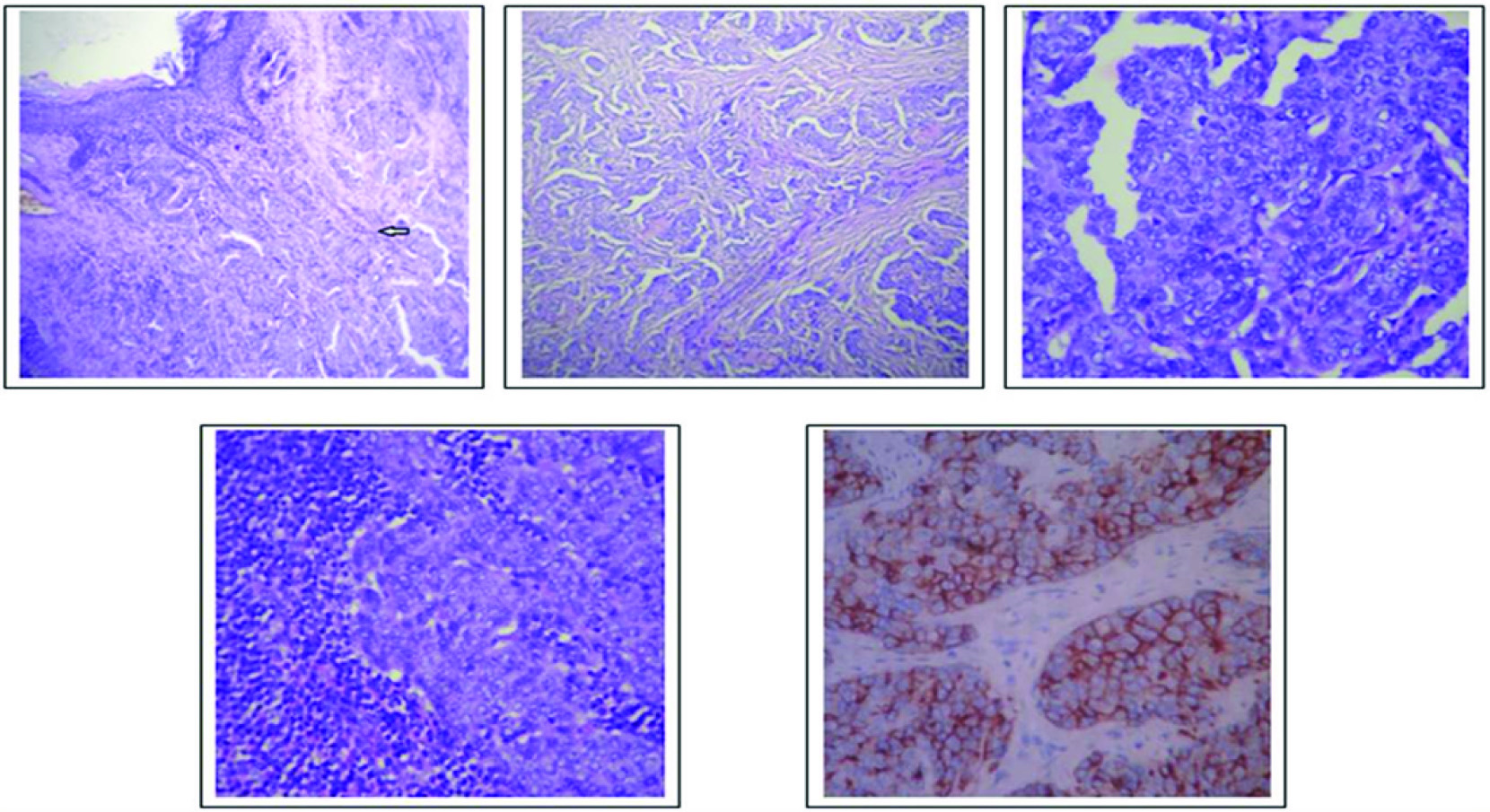
Histomorphology revealed hyperplastic stratified squamous epithelium with ulceration and an interconnected epithelial down growth of neoplastic basaloid cells arranged in nests, anastomosing cords, in insular pattern and solid columns extending into the dermis with areas showing epidermotropism [Table/Fig-4a-c]. Duct like structures interspersed between the tumour cells were seen. Scattered mitotic figures and focal perineural invasion was also present. Axillary lymphnodes showed metastatic carcinomatous deposit [Table/Fig-4d]. Immunohistochemical study with EMA showed diffuse membrane positivity [Table/Fig-4e] and CEA showed focal positivity. A diagnosis of Eccrine porocarcinoma of the right axilla with metastasis to axillary lymph nodes was given. Patient underwent postoperative radiotherapy and was followed up for a period of 12 months and showed no features of loco-regional recurrence.
Discussion
Eccrine porocarcinoma, a rare malignant sweat gland tumour, was first described by Pinkus and Mehregan as Epidermotropic eccrine carcinoma [1]. It was Mishima and Morika who ascribed the name Eccrine porocarcinoma [2]. This biologically aggressive tumour accounts for 0.005% of cutaneous neoplasms [3], and arises denovo or in a prexisting poroma or hydroacanthoma simplex. Rarely it may arise from an organoid nevus. The most common sites are the palms and soles in adults with no sex predilection.
Origin of this tumour is from the intraepidermal and dermal eccrine ducts. Clinically these tumours are slow growing and present as verrucous plaque or nodule to a polypoidal ulcerated lesion. It is often misdiagnosed clinically as seborrhoeic keratosis, pyogenic granuloma, viral wart or squamous cell carcinoma. It commonly occurs between the 6th to 7th decade of life and has equal sex incidence. The most commonest sites of predilection are the lower extremities (50%), trunk (24%) head and neck accounting to 18% [3,4]. Other sites of occurrence are upper extremities (2%) and hands (3%). So far 6 cases of eccrine porocarcinoma of vulva and penis have been reported. Our case was a 50-year-old male presenting with a verrucous lesion in the right axilla.
Suggested etiological factors for eccrine porocarcinoma are radiation therapy, solar damage and immunosuppression. The pathogenesis is still unknown but recently it has been proposed that p53 gene could be involved in the carcinogenesis of eccrine porocarcinoma [5]. Tumours with aggressive biological behaviour are those with infiltrative pattern of growth accompanied by a desmoplastic stromal reaction, apoptosis of tumour cells, high grade cytological atypia throughout the tumour, nucleolation, hyperchromasia, perineural invasion, vascular invasion and extensive clear cell change.
Cytology plays an important role in the diagnosis of primary cutaneous malignant epithelial tumours [6]. FNAC shows hypercellular smear with discohesive clusters, sheets and singly dispersed cells having illdefined to well defined cell borders, variable amount of vacuolated to dense and refractile cytoplasm and pleomorphic, hyperchromatic nuclei with coarse chromatin and occasional nucleoli. Binucleated and multinucleated forms can also be seen. The cytological differential diagnosis is basal cell carcinoma, nonkeratinizing squamous cell carcinoma, metastatic lobular breast carcinoma and malignant melanoma. The cytological features of basal cell carcinoma are tight clusters of small round to oval cells with peripheral palisading, ill-defined cell borders, minimal cytoplasm, high nucleo/cytoplasmic ratio and oval to spindled nuclei with inconspicuous nucleoli. Neoplastic cells in case of nonkeratinizing squamous cell carcinoma are arranged as two-dimensional geographic sheets or are seen in singles which have dense and refractile cytoplasm with well defined cell borders, hyperchromatic and pleomorphic nuclei with coarse chromatin and prominent nucleoli. Cytological features of metastatic lobular breast carcinoma show poorly cohesive cell clusters and single files of uniform small cells with hyperchromatic nuclei. In malignant melanoma, the neoplastic cells are spindled to epitheloid and are seen in singles and loose clusters. Intracytoplasmic melanin pigment, multinucleation, large eccentric nuclei with intranuclear inclusions are seen.
Grossly tumours vary from 1 to 10cm and appear nodular, infiltrative, ulcerated, polypoidal and asymmetrical. Multinodularity, ulceration and rapid growth are associated with local recurrence or metastases [7].
Histologically most of these tumours exhibit acanthotic epidermis with tumour cells invading into the dermis forming nests, sheets and cords with focal insular pattern. The tumour cells are polygonal or fusiform with round to oval pleomorphic nuclei with indistinct nuclear borders and variable amount of cytoplasm. Clear cells with round to oval nuclei and clear cytoplasm with distinct cell borders can be seen but are less prominent. Intracytoplasmic lumina formation and connection to the intradermal eccrine ducts may be seen. Intralymphatic invasion may be seen in the deeper dermis in 15% of cases. Interspersed duct like structures and irregular cystic spaces can be seen among the tumour cells and are a clinching point to the diagnosis. Other morphological appearance of tumour cells include spindle cells, mucous cell metaplasia, a pagetoid phenomenon and colonization by melanocytes [8–10]. Tumour with only intraepidermal component is known as in-situ porocarcinoma. Benign component of eccrine poroma or hydroacanthoma simplex may be seen in 10% cases. Keratinization is usually absent. The differentials for eccrine porocarcinoma are basal cell carcinoma, squamous cell carcinoma, amelanotic melanoma and metastatic adenocarcinoma. Malignant eccrine porocarcinoma can be distinguished from infiltrating basal cell carcinoma by the absence of peripheral palisading. Squamous cell carcinoma shows tumour cells with intercellular bridges and keratin pearls. Amelanotic melanoma is composed of spindled shaped cells and differentiation from carcinoma is quite difficult and hence correlation of findings with immunohistochemical markers is mandatory.
Neoplastic cells contain glycogen and intratubular PAS positive, diastase resistant material is usually present. Immunohistochemical markers such as pancytokeratin stains the tumour cells paler than the adjacent epidermal keratinocytes but EMA and CEA is found to stain the tumour cells strongly positive. Our case exhibited strong positivity for EMA and focal positivity for CEA.
An increased incidence of multiple cutaneous and visceral metastasis has been reported in eccrine porocarcinoma. High mitotic index, lymphovascular invasion, tumour size and depth greater than 7mm carry a poor prognostic outcome. Perineural invasion and lymphatic invasion was seen in the present case. Treatment modality for eccrine porocarcinoma is total surgical excision with broad tumour margins and regional lymph node dissection with a cure rate of 80%, but Moh’s micrographic surgery is found to be more valuable [11]. Recurrence rate in eccrine porocarcinoma is 20% and metatastasis to lymph nodes accounts for 20% approximately. A high mortality rate of 75-80% was documented in various case series [12]. Follow up of our case after surgery followed by chemo/radiotherapy showed no locoregional recurrence. A table showing a comparison of similar reported cases is provided [Table/Fig-5] [3,13–15].
(a) Interconnected epithelial downgrowth of neoplastic basaloid cells (H&E, 100X); (b) Porocarcinomatous cells in nests and insular pattern (H&E, 100X); (c) Pleomorphic polygonal cells with indistinct cell borders having pale cytoplasm and round to oval nuclei with prominent nucleoli (H&E, 400X); (d) Lymph node showing metastatic porocarcinomatous cells (H&E, 400X); (e) Diffuse membrane positivity EMA (400X).
Summary of eccrine porocarcinoma cases in literature
| Author | Age/Sex | Duration | Site | Size | Metastasis | Association with Eccrine Poroma |
|---|
| Kim et al., [13] | 86/F | 10 yrs | Right thigh | 5X4CM | Regional lymph nodes | Yes |
| Choi et al., [14] | 44/M | 7 Months | Right scrotum and pelvic area | 6X7CM | Regional lymph nodes | No |
| Chang O et al., [3] | 42/M | 6 yrs | Right lower limb | 3x2x 0.6cm | Absent | Yes |
| KA Vaidya [15] | 90/m | 2 yrs | Right forearm | 3.5 x 3x3cm | Absent | No |
| Present case | 50/M | 4 yrs | Right axilla | 4x3.5x 2.5cm | Lymph node metastasis | No |
Conclusion
Eccrine porocarcinoma, an intriguing tumour of indolent behaviour is still a challenge for surgeons and medical/radiation oncologist and hence pathological examination helps in arriving at a right diagnosis and to plan an appropriate treatment protocol. Close follow up is necessary to detect local recurrence and lymph node metastasis. A basic knowledge of this tumour has to be borne in mind while dealing with cutaneous lesions.
[1]. Pinkus H, Mehregan AH, Epidermotropic eccrine carcinomaArch Dermatol 1963 88:597-606. [Google Scholar]
[2]. Mishma Y, Morioka S, Oncogenic differentiation of the intra-epidermal eccrine sweat duct: eccrine poroma, poro-epithelioma and porocarcinomaDermatologica 1969 138:238-50. [Google Scholar]
[3]. Chang O, Elnawawi A, Rimpel B, Asarian A, Chaudhry N, Eccrine carcinoma of the lower extremity: A case report and review of literatureWorld J Surg Oncol 2011 9:94 [Google Scholar]
[4]. Vandewyer E, Renoirte C, Musette S, Gilles A, Eccrine Porocarcinoma : A Case ReportActa Chir Belb 2006 106:121-23. [Google Scholar]
[5]. Akalin T, Sen S, Yuceturk A, Kandiloglu G, P53 protein expression in eccrine poroma and porocarcinomaAm J Dermatopathol 2001 23:402-06. [Google Scholar]
[6]. Kalogeraki A, A cytologic approach of eccrine porocarcinomaActa Med Port 2013 26(4):467-70. [Google Scholar]
[7]. Robson A, Greene J, Ansari N, Eccrine porocarcinom (malignant eccrine poroma): a clinicopthologic study of 69 casesAm J Surg Pathol 2001 25:710-20. [Google Scholar]
[8]. Ribeiro LC, Luz MA, Montenegro MFG, Biasi LJ, Ogata DC, Eccrine Porocarcinoma (Malignant Eccrine Poroma) with Bone Invasion and Lymph Node MetastasesApp Cancer Res 2008 28:165-67. [Google Scholar]
[9]. Snow SN, Reizner GT, Eccrine porocarcinoma of the faceJ Am Acad Dermatol 1992 27:306-11. [Google Scholar]
[10]. Brown CW, Dy LC, Eccrine porocarcinomaY Derm Ther 2008 21:433-38. [Google Scholar]
[11]. Wittenberg GP, Robertson DB, Solomon AR, Washington CV, Eccrine Porocarcinoma treated with Mohs Micrographic Surgery: A Report of five casesDermatol Surg 1999 25:911-13. [Google Scholar]
[12]. Elder David E, Lever’s Histopathology of the Skin 1949 10th ed [Google Scholar]
[13]. Kim JS, Ro YS, Park CK, A case of malignant eccrine poromaKorean J Dermatol 1998 36:717-21. [Google Scholar]
[14]. Choi CM, Cho HR, Lew BL, Sim WY, Eccrine porocarcinoma presenting with unusual clinical manifestations: A case report and review of the literatureAnn Dermatol 2011 23(suppl 1):S79-83. [Google Scholar]
[15]. Vaidya KA, Shankarling M, Sukesh Eccrine Porocarcinoma of Skin: A Rare Case Report with Review of LiteratureSch J App Med Sci 2014 2(1A):125-27. [Google Scholar]