Juvenile Granulosa Cell Tumour: Anaplastic Variant with Omental Deposits
Anuradha C.K. Rao1, Manjari Kishore2, Vidya Monappa3
1 Professor, Department of Pathology, Kasturba Medical College, Manipal, Karnataka, India.
2 Junior Resident, Department of Pathology, Kasturba Medical College, Manipal, Karnataka, India.
3 Associate Professor, Department of Pathology, Kasturba Medical College, Manipal, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Manjari Kishore, Flat No 252, Type C, Old Valley Flats, KMC Campus, Manipal, Karnataka-576104, India.
E-mail: drmanjarik@gmail.com
Juvenile Granulosa Cell Tumour (JGCT) of ovary represents a small fraction of all primary ovarian malignancies. It is a subtype of granulosa cell tumour that is almost always found during the first three decades of life. Histologically, it differs from the typical adult type of granulosa cell tumour. It accounts for 5-15% of all granulosa cell tumours, majority being unilateral.
Herein, we describe an unusual histopathological variant of JGCT with numerous large cystic spaces, anaplasia and focal syncytiotrophoblast like giant cells.
Histopathology, Immunohistochemistry, Juvenile GCT, Ovarian malignancies
Case Report
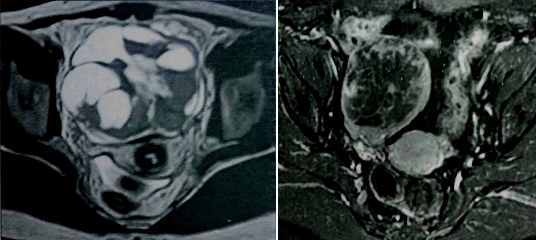
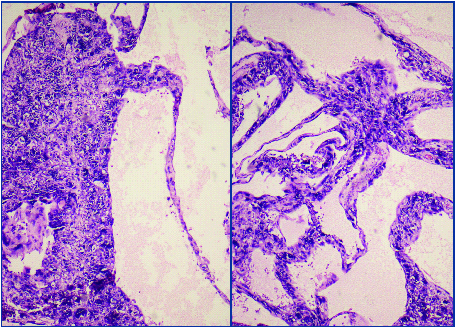
A 15-year-old female presented with history of enlarging abdominal mass, distension more on right side along with pain abdomen. Biochemical investigation showed normal liver function test and coagulation profile; β-HCG <0.1 mIU/mL, AFP- 2.6 ng/mL, testosterone level- 0.1 ng/mL, 24-hr urine ketosteroid- 3.7 mg/ 24 hours. Ultrasound showed an abdominopelvic heterogeneous mass measuring 20.4cm X 19.8 cm. CT scan showed multiloculated, heterogeneous right ovarian cyst with bilateral hydrouereteronephrosis. MRI done showed large muticystic mass with some solid portion [Table/Fig-1,2]. Contralateral ovary and uterus were normal.
MRI images of tumour showing large multicystic mass with some solid portion.
Patient was subjected to right ovarian cystectomy and tumour sent for histopathological examination.
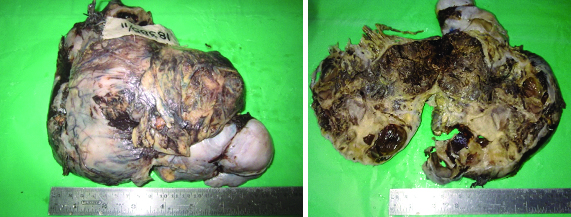
Grossly, a large intact ovarian tumour was received, measuring 22.5 cm X 8.5 cm X 7 cm and weighed 1782 gram. The surface was bosselated and composed of grey-white fibrous capsule [Table/Fig-3]. Cut section [Table/Fig-4] showed predominantly solid variegated, grey white areas with focal haemorrhagic, grey tan areas and yellow areas. Multiple cystic spaces filled with mucoid material was also noted.
Gross images of the tumour (External surface & cut surface).
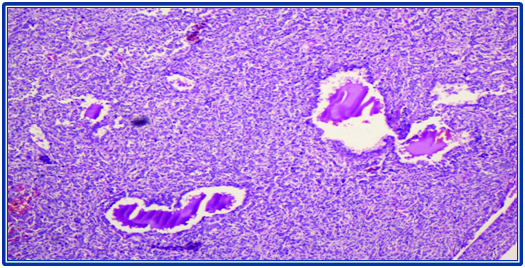
Multiple sections from the ovarian mass showed a tumour composed of sheets of polygonal cells with focal luteinization arranged in alveolar, macrofollicular and focal glandular pattern [Table/Fig-5,6]. Individual cells showed moderate clear to amphophilic cytoplasm, enlarged irregular vesicular nuclei, single prominent nucleoli [Table/Fig-7]. Scattered intracellular and extracellular eosinophilic hyaline globules and solid spindle areas were noted. Brisk mitotic figures seen along with areas of haemorrhage and extensive necrosis. Areas with cystic change, numerous syncytiotrophoblast like bizarre uninucleate and multinucleate giant cells were noted [Table/Fig-8]. Omental tumour deposits were also seen.
Microscopic image showing the alveolar pattern of tumour with macrofollicles (H&E: 100X).
High power view of the alveolar pattern (H&E:200X).
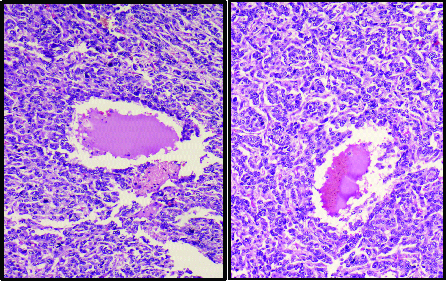
Microscopic image showing sheets of polygonal cells with clear cell change (H&E:200X).
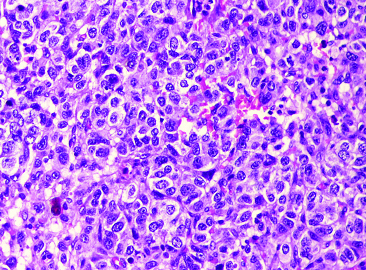
Fig showing cystic pattern with adjacent syncytiotrophoblastic like cells (H&E:100X).
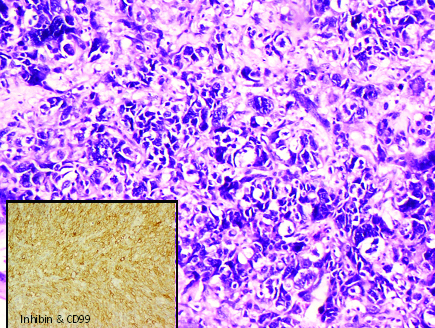
Immunohistochemically non-anaplastic areas of tumour showed diffuse cell membrane positivity for CD99 and focal positivity for inhibin [Table/Fig-9]. CK7 was negative. Finally, a diagnosis of: Juvenile Granulosa Cell Tumour of Right Ovary: Anaplastic variant with omental deposits (Stage III). On follow up of the patient, it was noted that patient had been treated with four cycles of combination chemotherapy (BEP). The patient was found well tolerated to these drugs. In recent scan, no abnormality was found in abdomen or pelvis and the contralateral (left) ovary and uterus was visualized, being normal in size and echotexture.
Syncytiotrophoblastic like cells with anaplasia (H&E: 200X) and IHC with Inhibin showing focal positivity (inset).
Discussion
Ovarian cancer is the third most common neoplasm of the female gynecologic tract, after carcinoma of cervix and endometrium. It accounts of approximately 6% of all cancers found in female. Most of the cases with ovarian malignancy present in advanced-stage disease [1]. On the basis of type of cell found, primary ovarian malignancies are classified in surface epithelium, germ cell or sex cord tumours [2]. Sex cord tumours account for 1% to 2% of ovarian malignancies. Approximately, 70% of sex cord tumours are granulosa cell tumours, being hormonally active tumours. If theca cells (luteinized cells) are present in large quantities within a granulosa cell tumour, there can be elevated serum estradiol levels. Among the histologic subtypes of granulosa cell tumour (GCT), juvenile granulosa cell tumours are rare [3], typically presenting early in life and majority of cases are unilateral. It was observed in a study that of 125 patients with JGCT, 78% presented in first two decades of life.
As far as the aetiology is concerned, the exact cause is unknown. In few studies, two modifier genes have been mapped in chromosome X of mice supporting the development of juvenile GCT in female mice [4].
It is observed that 80% of JGCT occurs in children, which comes to attention because of isosexual pseudoprecocity (precocity without ovulation or progesterone production). The patients presenting after puberty usually come with menstrual irregularities, amenorrhea, abdominal pain and swelling. Sometimes, ascites, tumour rupture, torsion, infarction can be noted in tumours. JGCT can present with some other conditions like Tuberous sclerosis, nonhereditary congenital syndromes like Ollier’s diseases, Maffuci syndrome. Bilateral presentation can be seen in Goldenher and Potter syndrome.
Grossly, appearance of JGCT is not distinctive and is quite similar to adult type. JGCT, typically presents as a solid cellular, with focal variably sized follicle formation and usually the follicles formed are not as large as seen in follicular variant of adult type [5].
Eosinophilic or basophilic fluid can be noted in the lumen. Neoplastic cells in solid areas may be arranged diffusely or divided in lobules by fibrous septae. Sometimes, luteinization can be so much that follicles are reduced in number and poorly developed. The individual tumour cells have abundant eosinophilic and/or vacuolated cytoplasm and rounded hyperchromatic nuclei. Mitotic rate is higher than in adult variant. The diagnosis of GCT can be confirmed using immunohistochemical markers like inhibin, CD99, Calretinin. Markers like EMA, keratin, MART-1 are usually negative [5].
Serum inhibin levels are useful in follow up of these patients. In contrast to Adult GCT, which recur late, almost all clinically malignant JGCT recur within 3 years [6]. Recurrences are treated with cisplatin-based chemotherapy with some success.
JGCT can mimic many other ovarian neoplasms, both clinically and histologically. Owing to the presence of precocious puberty, it can mimic any of the other sex cord stromal tumours clinically; in which case, HPE provides confirmatory diagnosis [7]. Microscopically, and in the absence of hormonally induced symptoms, it could mimic any of the epithelial malignancies, germ cells tumours or Small cell neuroendocrine carcinoma. In recent years several IHC markers identifying JGCT have become commercially available, which is of value in distinction between tumours which mimic JGCT. The differential diagnosis which can be considered in this case are summarized in [Table/Fig-10].
Table showing Differential Diagnosis of Juvenile Granulosa Cell Tumour with characteristic features.
| Feature | JGCT | AGCT | Embryonal carcinoma | Yolk sac tumour | Melanoma prim & metastasis | Small cell NEC | Surface epithelial ca |
|---|
| Clinical feature | <30 y (avg 13 y) | 1st to 10th decade(median 50 y)Late recurrences | U/LMedian age 15 y | U/L<3 y | B/L ovarian involvementPrior H/O melanoma | Aggressive tumours in young femalesAssociated with hypercalcaemia | Rare in young age group |
| Gross | Solid, cystic, lobulated areasFocal haemorrhagic areas | Frequent areas of haemorrhage | Solid, necrotic & haemorrhagic areas | Smooth necrotic & haemorrhagic areas | | Undifferentiated uniform, small, hyperchromatic nuclei with brisk mitosisForm follicular structures with eosinophilic materialCells with scanty cytoplasm distributed in sheets | |
| HPE | Polygonal cells with clear to amphophilic cytoplasmCall exner bodies rare | Follicles less irregular in size & shapeMyxoid background & luteinization less commonNuclear grooves & call exner bodies +nt | Primitive nucleiPattern solid, papillary & glandular with necrosis | Primitive nucleiPattern- reticular, glandularSchiller duval bodies | Melanin pigment | | |
| IHC | CD99 +ve, focal inhibin +ve,CK7 -ve | EMA-ve,CD99 & vimentim +ve | CD30, SALL4, Oct4 & HCG +veInhibin -ve | AFP, Glypican 3, SALL4 +veInhibin –ve | Prominent expression of melanocytic markers | Inhibin –veCytokeratin & synaptophysin +ve | |
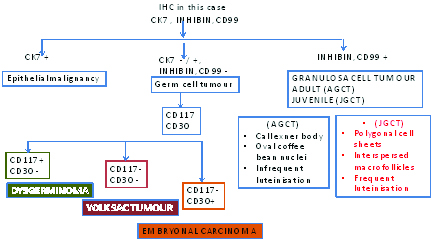
In the present case, the importance of immunohistochemistry lies in its implication in differentiating the JGCT from other ovarian tumours. This has been demonstrated in a flowchart as show in [Table/Fig-11].
Flowchart showing implication of Immunohistochemistry in the present case.
Conclusion
JGCT of ovary represent a very small fraction of all primary ovarian malignancy. They are typically seen within the first two decades of life with signs of hyperestrogenism and abdominal mass. If Juvenile GCT is diagnosed in early stages, it has favourable prognosis, and behaves less aggressively. Conclusive diagnosis requires HPE & IHC. More than 90% survival rate for patients with stage Ia tumours. Advanced staged tumours have been successfully treated with Platinum based combination chemotherapy (BEP). As there is a possibility of tumour recurrence, patients should be kept under long-term follow and surveillance.
[1]. Mount SL, Eltabbakh GH, Cooper K, Recent "non-gynecological" immunohistochemical markers in diagnostic ovarian pathologyCurr Diagn Pathol 2003 9:11-18. [Google Scholar]
[2]. Roth LM, Recent Advances in the Pathology and Classification of Ovarian Sex Cord-Stromal TumoursInt J Gynaecol Path 2006 25:199-215. [Google Scholar]
[3]. McCluggage WG, Immunohistochemical and Functional Biomarkers of Value in Female Genital Tract LesionsInt J Gynaecol Path 2006 25:101-20. [Google Scholar]
[4]. Scully RE, Young RH, Clement PB. Atlas of Tumour Pathology: Tumours of the Ovary, Maldeveloped Gonads, Fallopian Tube, and Broad Ligament. AFIP Fascicle 23, Third Series; 1996 [Google Scholar]
[5]. Young RH, Sex cord-stromal tumours of the ovary and testis: their similarities and differences with consideration of selected problemsMod Pathol 2005 18(Suppl 2):S81 [Google Scholar]
[6]. Quirk JT, Natarajan N, Ovarian cancer incidence in the United States, 1992-1999Gynecol Oncol 2005 97:519 [Google Scholar]
[7]. McCluggage WG, Recent advances in immunohistochemistry in the diagnosis of ovarian neoplasmsJ Clin Pathol 2000 53:327 [Google Scholar]