Chloramphenicol – A Potent Armament Against Multi-Drug Resistant (MDR) Gram Negative Bacilli?
Smita Sood1
1 Senior Microbiologist, Department of Laboratory Medicine (SRL Ltd.), Fortis Escorts Hospital, Jaipur, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Smita Sood, 3Kha 4A, Jawahar Nagar, Jaipur-302004, India. E-mail : drsmitasood@yahoo.co.in
Introduction
Multidrug-resistant gram-negative bacteria cause infections which are hard to treat and cause high morbidity and mortality. Due to limited therapeutic options there is a renewed interest upon older antimicrobials which had fallen into disuse as a result of toxic side effects. One such antibiotic is chloramphenicol which was sidelined due to reports linking its use with the development of aplastic anaemia.
Aim
A study was conducted to evaluate the susceptibility of chloramphenicol in light of the emerging problem of multi-drug resistant gram negative bacteria (MDR GNB).
Materials and Methods
A total of 483 MDR GNB of the 650 consecutive Gram Negative Bacteria isolated from various clinical samples of patients admitted at a tertiary care hospital in Jaipur between January-June 2014 were screened for chloramphenicol susceptibility by the disc diffusion method as per CLSI guidelines.
Results
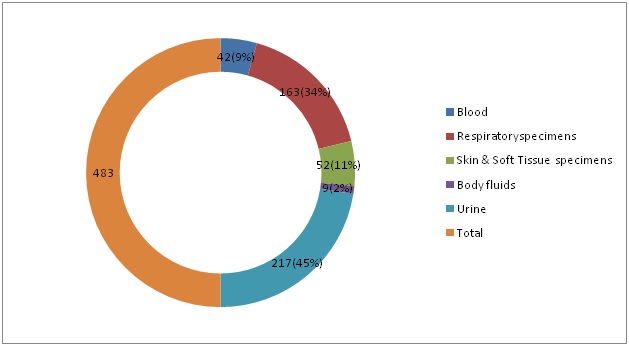
The MDR GNB isolates were obtained from 217 (45%) urine, 163 (34%) from respiratory samples, 52(11%) from pus, 42 (9%) from blood and 9 (2%) from body fluids. A 68% of the MDR GNB isolates were found to be sensitive to chloramphenicol.
Conclusion
Clinicians should always check for the local susceptibility of Gram-negative bacteria to chloramphenicol. This antibiotic has a potential to play a role in the therapeutic management of infections due to MDR GNB pathogens.
Antimicrobials, Disc diffusion method, Gram negative bacteria
Introduction
Antibiotic resistance has become a major clinical and public health problem within the lifetime of most people living today. The World Health Organization has declared antibiotic resistance as a major threat to global health security. Compared to the developed countries, the ease of availability and inappropriate use of antibiotics in developing countries have resulted in far greater levels of antibiotic resistance [1].
Multidrug resistance (MDR) is defined as non-susceptibility to one or more antimicrobials on three or more antimicrobial classes, while strains that are non-susceptible to all antimicrobials, are classified as extreme drug-resistant strains [2]. The rising incidence of Multidrug-resistant Gram negative bacilli (MDR-GNB) presents a challenge in healthcare settings because of the paucity of effective antimicrobial agents that are available to treat infections with these organisms. Furthermore, no new research antimicrobial molecules having an activity solely against gram-negative spectrum or bacteria already resistant to all currently available antibacterials are under development [3,4].
This shifts our focus to reexamine older antibiotics that had largely been banished because of their toxic side effects. One such agent is chloramphenicol which was released for use in the United States in 1949. The impulse to reexamine this older antibiotic is all the stronger because it has not been heavily used in recent years, thereby not giving the bacteria much chance to develop resistance.
It was against this background, this study was conducted to assess the sensitivity of chloramphenicol against multidrug resistant gram negative bacteria isolated from patients at a tertiary care hospital in Jaipur.
Materials and Methods
A total of 650 consecutive Gram Negative Bacteria were isolated in the Microbiology lab from various clinical samples of patients admitted at a tertiary care hospital in Jaipur, Rajasthan between January - June 2014. The organism identification and sensitivity testing was routinely performed on an automated system (Microscan autoScan- 4 by Seimens Healthcare Ltd.). For the purpose of the study, multidrug resistance was considered as resistance to at least three of the different classes of commonly used antimicrobials (Penicillins, Cephalosporins, Aminoglycosides, Flouroquinolones, β lactams/β lactamase inhibitor combinations and Carbapenems). Consecutive MDR GNB isolates were further screened for chloramphenicol susceptibility by the disc diffusion method as per CLSI guidelines [5]. The isolates with a zone diameter measuring ≤ 12 mm were considered as resistant, those with zone diameter measuring between 13-17mm were considered as intermediate and those with zone diameter measuring ≥ 18 mm were considered as sensitive.
Results
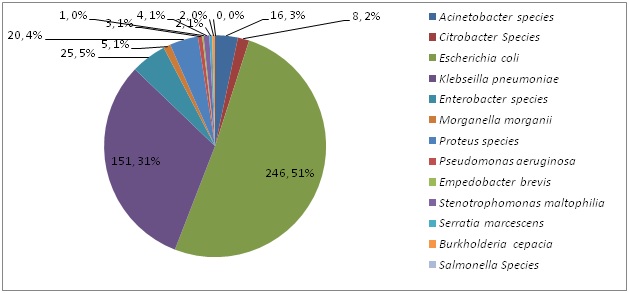
A total of 483 (75%) MDR-GNB strains were obtained from 650 consecutive GNB isolated in the Microbiology lab during the six months study period. [Table/Fig-1] shows the percentage isolation of MDR GNB isolates from various clinical specimens. The percentage isolation of MDR - GNB was highest for urine (45%) followed by respiratory specimens (34%) and pus specimens (11%). [Table/Fig-2] shows the frequency distribution of MDR - GNB. Majority of the MDR-GNB isolates were Escherichia coli (51%) and Klebseilla pneumoniae (31%). [Table/Fig-3] shows the percentage sensitivity of Chloramphenicol to various MDR-GNB. A total of 68% of MDRGNB were found to be sensitive to Chloramphenicol.
Percentage Isolation of MDR-GNB isolates from various clinical specimens
Frequency distribution of Multi-drug Resistant Gram Negative Bacteria
Percentage Sensitivity of Chloramphenicol to various Multi-drug Resistant Gram Negative Bacteria
| Gram Negative Bacteria | MDR-GNB Isolates sensitive to chloramphenicol/Total MDR-GNB isolates (% Sensitivity of MDR GNB isolates to Chloramphenicol) |
|---|
| Acinetobacter baumannii | 3/16(19) |
| Citrobacter Species | 5/8(62) |
| Escherichia coli | 204/246(83) |
| Klebseilla pneumoniae | 77/151(51) |
| Enterobacter species | 18/25(72) |
| Morganella morganii | 5/5(100) |
| Proteus species | 7/20(35) |
| Pseudomonas aeruginosa | 1/3(34) |
| Empedobacter brevis | 1/1(100) |
| Stenotrophomonas maltophilia | 3/4(75) |
| Serratia marcescens | 1/2(50) |
| Burkholderia cepacia | 2/2(100) |
| Total | 483/650(68) |
Discussion
The alarming epidemic of MDR bacteria and the reluctance of the pharmaceutical industry to invest in the development of new antibiotics have forced the clinicians to reintroduce forgotten antibiotics in clinical practice. Chloramphenicol is one such old broad-spectrum antibiotic.
This antibiotic was originally derived from the bacterium Streptomyces venezuelae. It was isolated by David Gottlieb and introduced into clinical practice under the trade name Chloromycetin and was widely used in the 1950s [5]. Chloramphenicol is a potent inhibitor of protein synthesis and acts by binding reversibly to the 50S subunit of the bacterial ribosome and is extremely active against a variety of organisms including bacteria, spirochetes, rickettsiae, chlamydiae and mycoplasmas. It has bacteriostatic activity against most pathogens but is bactericidal for Haemophilus influenzae, Streptococcus pneumoniae, and Neisseria meningitides [6,7]. It has good oral bioavailability and excellent tissue penetration. However, its use in the developed countries was abandoned due to reports linking this drug with serious adverse effects such as irreversible and fatal aplastic anaemia and gray baby syndrome in premature and newborn infants [8].
After decades of limited use, this drug is now the focus of renewed interest. Because of the ease of availability and low cost Chloramphenicol is still widely used in many developing countries to treat typhoid fever, anaerobic infections, bacterial meningitis in patients allergic to penicillin, brain abscesses and rickettsial infections [9]. The data on chloramphenicol susceptibility patterns in developed countries in recent years is limited. In India, studies have documented a 90–95% re-emergence of chloramphenicol susceptibility among Salmonella enterica serotype typhi isolates [10,11].
We identified 483 (74%) MDRGNB strains from 650 consecutive GNB isolated in the Microbiology lab during the study period. A total of 68% of MDRGNB were found to be sensitive to Chloramphenicol in our study. A similar study conducted on 1037 consecutive MDRGNB isolates from ICU admitted patients at a level - 1 trauma centre at AIIMS, New Delhi found only 15% (155) of the isolates to be susceptible to chloramphenicol [12]. Another study from a tertiary care hospital in Tirupati, South India reported 25.5% (40/157) of the pan drug resistant GNB to be sensitive to Chloramphenicol [13]. The probable explanation for a much higher chloramphenicol sensitivity observed compared to the two previous Indian studies is a totally different geographical location (West India) where the present study was conducted. Further the two Indian studies were conducted a few years back and the time gap could have contributed to resurgence of chloramphenicol sensitivity in our region. Moreover, the fact that Chloramphenicol consumption is minimal at our hospital would also explain for higher sensitivity of the drug at our centre.
This study reveals that 83% of the MDR Escherichia coli isolates and 50% of the MDR Klebseilla pneumoniae isolates to be sensitive to chloramphenicol. In a recent National survey conducted at Israeli hospitals on Chloramphenicol use and susceptibility patterns, the susceptibility of Enterobacteriaceae ranged between 73%-90% to chloramphenicol [9]. In a study from Surat, Gujarat only 7.6% of the Enterobacteriaceae was found to be sensitive to chloramphenicol [14]. Nitzan et al., have reported a lower resistance rate to chloramphenicol (18.4%) in all the members of the Enterobacteriaceae, reaching statistical significance in E. coli, Klebsiella spp., Enterobacter spp., Citrobacter spp., Serratia spp. and Morganella spp [15].
We found 19% of MDR Acinetobacter baumannii and 33% of the MDR Pseudomonas aeruginosa strains to be sensitive to Chloramphenicol. Our findings are consistent with a recent review which reported susceptibility rates of the non-fermenting pathogens A. baumannii and P. aeruginosa to chloramphenicol varying from 0 -20% [15].
Hussain et al., from Pakistan have reported 71.7% of the ESBL GNB isolates to be sensitive to chloramphenicol [16]. Another study carried out in United Kingdom revealed efficacy of chloramphenicol against Carbapenem resistant GNB [17].
A recent systematic literature review of 113 studies on the invitro activity of chloramphenicol against clinical ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumanii, Pseudomonas aeruginosa, Enterobacter spp.) found high susceptibility rates among gram-positive bacteria. The authors concluded that though the risks versus the benefits of chloramphenicol use are yet to be analyzed but the role of chloramphenicol needs to be re-examined in light of the emerging problem of multi-drug-resistant pathogens [18].
In another recent systematic review and meta-analysis of randomized controlled trials (RCTs) on the efficacy and safety of chloramphenicol, it was concluded that Chloramphenicol is a safe alternative antibiotic for shorter antibiotic regimens but in comparison to the available alternatives its efficacy is not established for the treatment of respiratory tract infections, meningitis and enteric fever. Further, more of RCTs are needed for evaluating the use of chloramphenicol for the treatment of MDR organisms in absence of alternative antibiotics [19].
Limitation
The main limitation of our study is that it shows results from a restricted geographic area - a single tertiary care centre and is purely laboratory based. Moreover; there was no clinical correlation to see the therapeutic outcomes of the drug. It would be more beneficial if multicentric studies are carried out to find out the susceptibility of MDRGNB isolates against chloramphenicol.
Conclusion
In this era of increasing antibiotic resistance and in view of relatively high susceptibility rates, Chloramphenicol may have an expanded role in our antimicrobial arsena. It becomes important that we reacquaint ourselves with this potent antimicrobial. There is need to further evaluate this antimicrobial for determining the in vitro as well as invivo efficacy before broad based usage of this compound can be undertaken.
[1]. Kumar SG, Adithan C, Harish BN, Sujatha S, Roy G, Malini A, Antimicrobial resistance in India: A reviewJ Nat Sci Biol Med 2013 4(2):286-91. [Google Scholar]
[2]. Kallen AJ, Srinivasan A, Current epidemiology of multidrug-resistant Gram-negative bacilli in the United StatesInfect Control Hosp Epidemiol 2010 31(Suppl 1):S51-54. [Google Scholar]
[3]. Lautenbach E, Polk RE, Resistant gram-negative bacilli: A neglected healthcare crisis?American Journal of Health-System Pharmacy 2007 64((23) Suppl 14):S3-S21. [Google Scholar]
[4]. Xu ZQ, Flavin MT, Flavin J, Combating multidrug-resistant Gram-negative bacterial infectionsExpert Opin Investig Drugs 2014 23(2):163-82. [Google Scholar]
[5]. Madhavan HN, Bhagyalakshmi R, Farewell, Chloramphenicol? Is this True? : A Review. Research and ReviewsJournal of Microbiology and Biotechnology 2014 3(1):13-26. [Google Scholar]
[6]. Howard J, Chloramphenicol - A ReviewPediatrics in Review 2004 25:284-88. [Google Scholar]
[7]. Mir SA, Abbas Z, Chloramphenicol: A come backJK Science 2010 12:153 [Google Scholar]
[8]. Cassir N, Rolain J - M, Brouqui P, A new strategy to fight antimicrobial resistance: the revival of old antibioticsFront Microbiol 2014 5:551 [Google Scholar]
[9]. Nitzan O, Kennes Y, Colodner R, Saliba W, Edelstein H, Raz R, Chloramphenicol Use and Susceptibility Patterns in Israel: A National SurveyIsr Med Assoc J 2015 17:27-31. [Google Scholar]
[10]. Khandeparkar P, Reemergence of Chloramphenicol in Typhoid Fever in the Era of Antibiotic ResistanceJAPI 2010 58:45 [Google Scholar]
[11]. Kumar Y, Sharma A, Raju KM, Re-emergence of susceptibility to conventionally used drugs among strains of Salmonella Typhi in central west IndiaJ Infect Dev Ctries 2011 5(3):227-30. [Google Scholar]
[12]. Mathur P, Behera B, Chaturvedi D, Misra MC, Chloramphenicol: Is Old Really Gold?JAPI 2010 58:384 [Google Scholar]
[13]. Jayaprada R, Chaudhury A, Venkataramana B, Shobha Rani A, Pan-resistance among gram-negative clinical isolates at a tertiary care hospital in South IndiaJ Clin Sci Res 2012 3:121-25. [Google Scholar]
[14]. Mulla S, Charan J, Panvala T, Antibiotic sensitivity of Enterobacteriaceae at a tertiary care centre in IndiaChron Young Sci 2011 2:214-18. [Google Scholar]
[15]. Nitzan O, Suponitzky U, Kennes Y, Kennes Chazan B, Raz R, Is chloramphenicol making a comeback?Isr Med Assoc J 2010 12:371-74. [Google Scholar]
[16]. Hussain A, Mirza IA, Ikra A, Sattar A, Ali S, Khan IU, Invitro Sensitivity of Chloramphenicol against Extended Spectrum Beta Lactamase Producing Gram Negative BacteriaInfectious Diseases Journal of Pakistan 2012 21(4):503-06. [Google Scholar]
[17]. Livermore DM, Warner M, Mushtaq S, Doumith M, Zhang J, Woodford N, What remains against carbapenem-resistant Enterobacteriaceae? Evaluation of chloramphenicol, ciprofloxacin, colistin, fosfomycin, minocycline, nitrofurantoin, temocillin and tigecyclineInt J Antimicrob Agents 2011 37(5):415-59. [Google Scholar]
[18]. Ivljak R C, Giannella M, Bella SD, Petrosillo N, Could chloramphenicol be used against ESKAPE pathogens? A review of in vitro data in the literature from the 21st centuryExpert Rev Anti Infect Ther 2014 12(2):249-64. [Google Scholar]
[19]. Eliakim-Raz N, Lador A, Leibovici-Weissman Y, Elbaz M, Paul M, Leibovici L, Efficacy and safety of chloramphenicol: joining the revival of old antibiotics? Systematic review and meta-analysis of randomized controlled trialsJ Antimicrob Chemother 2015 70(4):979-96. [Google Scholar]