Bronchial asthma is a chronic inflammatory disorder, mediated by many inflammatory pathways involving the interleukin, eosinophils, mast cells. Bronchial asthma is a T-helper cell mediated disorder, characterized by eosinophilic infiltration, bronchial hyper reactivity and hyper secretion of mucus by goblet cells [1]. Further structural changes in airway may lead to airway remodeling, goblet cell metaplasia, smooth muscle hyperplasia and hypertrophy [2,3]. Induced sputum and exhaled air are some of the methods to obtain airway samples. Apart from these methods, various biomarkers can also be measured to diagnose eosinophilic inflammation [4]. However, only few markers can be assessed easily in clinical settings, which reflect Th2 mediated eosinophilic inflammation. Periostin may be one such biomarker. Periostin is an extracellular matrix protein originally isolated from an osteoblast cell line and it is induced by IL-4 and IL-13 in airway epithelial cells [5,6]. However, there is a lack of evidence for the use of periostin as a serum biomarker for eosinophilic airway inflammation in South Indian asthma patients. With this background, the aim of the study is to determine the sensitivity and specificity of serum periostin as a non-invasive bio marker in asthmatic patients and to correlate the levels of sputum cells (eosinophils) and blood eosinophils in asthmatic subjects with serum periostin.
Materials and Methods
The study was conducted in the Pulmonary Medicine Department, SRM Medical College Hospital and Research Centre. The study was conducted over a period of 18 months from February 2013 to August 2014. The study was designed as a prospective, case-controlled study. Patients who presented with consistent symptoms of asthma and confirmed by spirometry with reversibility were the cases. Controls were the healthy subjects who had no history of lung disease with normal FEV1 (>80% predicted) on spirometry. Controls were recruited from patients bystanders. Institutional Ethics Committee (IEC) approval was obtained prior to the commencement of the study (Ethical Clearance Number: 442/IEC/2013). Written informed consent was obtained from all the study participants.
The study participants were aged between 18 to 60 years, both gender, clinically diagnosed moderate to severe persistent asthma, improvement in FEV1 greater than 12% after bronchodilator inhalation and patients who gave their written informed consent were included in the study. Patients were excluded if they had clinically significant renal, respiratory (other than asthma), cardiac, gastrointestinal, hepatic, endocrine disorders, haematological disorders, cancer, pregnant and lactating women, patients who underwent any major surgery. These inclusion and exclusion criteria were similar to our earlier studies carried out to assess the various second-line medications in addition to inhaled corticosteroid in asthma patients [1,7].
All the patients’ baseline demographic details and a detailed case history with special reference to upper airway symptoms, exposure to pet animals, smoking history, use of inhaled medications (type, dose, duration) any family history of sensitization were collected, followed by a systematic clinical examination. Spirometry testing was performed. Blood samples were collected and total counts, differential counts, absolute eosinophil counts, serum periostin were measured. Sputum samples were collected in a container in a sputum collection room and sent to laboratory for checking eosinophil counts. Similarly spirometry was performed in healthy volunteers, blood and sputum samples were collected and processed in the same manner. No formal sample size was calculated as this was a pilot study. With the consultation of biostatistician, in case and control groups, patients were taken in a ratio of 1:2 and a number not less than 30 and 60 in the case and control group respectively.
Pulmonary Function Test
Hand held spirometer (Mini Wrights peak flow meter,) was used in our study. The spirometry was performed in our Out-Patient department by trained technicians according to standardized American Thoracic/European Respiratory society criteria.
Human Periostin Elisa Test
Principle of the assay [
3,
5,
6]
The assay used in our study was sandwiched enzyme linked immuno-sorbent assay. An antibody for periostin has been pre-coated onto a microwell plate. Standards and samples were transferred to the microwells. After washing any unbound substances, a polyclonal antibody specific for periostin was added to the wells. Following washing, an ARIG-HRP conjugate was added to the wells. After washing any unbound enzyme, a substrate was added to the wells and colour developed in proportion to the amount of periostin bound in the initial step, which correlates with the intensity of the colour.
Plate preparation
Ninety six well microplate was coated with 100μL per well of the diluted capture antibody. The plate was sealed and incubated overnight at room temperature. Each well was aspirated and washed with wash buffer and this process was repeated two times for a total of three washes. Each well was washed again by filling each well with wash buffer (400μL) using a squirt bottle, manifold dispenseror auto-washer. Complete removal of liquid at each step was essential for good performance. After the last wash, any remaining wash buffer was removed by aspirating or by inverting the plate and blotting it against clean paper towels.
A 100μL of sample or standard in reagent diluents was added per well and covered with an adhesive strip and incubated for two hours at room temperature. The aspiration was repeated as mentioned in plate preparation. A100μL of the detection antibody was added and diluted in reagent diluent to each well and covered with a new adhesive strip and incubated for two hours at room temperature. Again the aspiration procedure was repeated as mentioned in plate preparation, 100μL of Streptavidin-HRP, the working solution was added to each well and incubated for 20 mins at room temperature. A 50μL of stop solution was finally added to all the wells and the optical density was determined at 450nm in a microplate reader.
Statistical Analysis
Statistical analysis was done by using software SPSS version 14 windows 8. Chi-square test was used to analyse the results (sensitivity and specificity). ROC analysis was done to calculate the cut off for periostin and AEC levels. Pearson correlation was performed to compare between variables. Quantitative data were expressed as mean (SD). A p<0.05 was considered to be statistically significant. Per protocol analysis was performed.
Results
Totally 101 cases were included in the study, in which 71 patients were moderate to severe persistent asthmatics and 30 were healthy controls with normal PFT. Baseline characteristics such as age, gender, upper respiratory symptoms, smoking history, family history and pet exposure are shown in [Table/Fig-1]. For all the 101 cases, sputum eosinophils, blood AEC levels and serum periostin levels were measured according to a standardized protocol. Out of the 71 asthma patients, 36 were male and 35 were female. In the control group, equal number of male and female cases were taken. The average age of the study participants was 44.31 years. Approximately 45% of the cases had a family history of atopy, which is a confounding factor for the development of asthma.
Baseline characteristics of study population.
| Demographic details | Frequency | Percentage |
|---|
| Age |
| < 30 years | 17 | 23.9 |
| 31 – 40 years | 13 | 18.3 |
| 41 – 50 years | 14 | 19.7 |
| 51 – 60 years | 15 | 21.1 |
| > 60 years | 12 | 16.9 |
| Male | 36 | 50.7 |
| Female | 35 | 49.3 |
| Upper airway symptoms |
| None | 19 | 26.8 |
| Nasal block | 1 | 1.4 |
| Sneezing | 2 | 2.8 |
| Sneezing and nasal block | 49 | 69.0 |
| Smoking history, n (%) |
| Smoker | 6 | 8.5 |
| Ex smoker | 2 | 2.8 |
| Non smoker | 63 | 88.7 |
| Pet exposure |
| None | 64 | 90.1 |
| Cat | 2 | 2.8 |
| Dog | 4 | 5.6 |
| Cat and dog | 1 | 1.4 |
| Family history |
| Positive | 32 | 16.1 |
| Negative | 39 | 54.9 |
Almost 81% of the cases had allergic rhinitis associated with asthma. Among the 71 cases, 31 were positive for sputum eosinophils. Twenty seven patients were not on any inhaled medications, 15 patients were on inhalers and the rest of 29 patients were on regular treatment with long acting beta agonists and inhaled corticosteroid combination. Descriptive analyses of the study population are shown in [Table/Fig-2].
Descriptive analysis of study population.
| Demographic details | Mean (SD) | 1st Quartile | 3rd Quartile |
|---|
| Age in years | 44.31 (15.46) | 31 | 57 |
| FEV1 / FVC | 58.95 (9.00) | 55 | 66 |
| Total count cells/cu.mm | 9830.14 (3030.59) | 8100 | 11600 |
| Neutrophils % | 74.01 (100.13) | 54 | 70 |
| Lymphocytes % | 26.65 (10.31) | 20 | 33 |
| Eosinophils % | 7.18 (6.92) | 3 | 10 |
| Monocytes % | 4.36 (1.84) | 3 | 5 |
| Basophils % | 1 (0) | 1 | 1 |
| Post % FEV1 | 64.43 (11.81) | 55 | 75 |
| Pre % FEV1 | 48.36 (11.96) | 39 | 60 |
| AEC cells/cu.mm | 751.89 (848.20) | 280 | 900 |
| Sr. periostin ng/ml | 45.20 (19.11) | 30.81 | 49.25 |
Sr. Periostin = Serum Periostin, FEV1= Forced Expiratory Volume in one second, AEC = Absolute Eosinophil counts
The PFT with reversibility of> 12% and 200ml, according to GINA guidelines, was taken as gold standard for detection of moderate and severe asthma cases. Independent samples t-test were performed to compare mean serum periostin values between normal PFT and obstructed (moderate and severe) PFT levels. This is to find the best cut-off value for serum periostin to detect the PFT obstruction, [Table/Fig-3] shows that serum periostin has a significant correlation with the severity of airflow obstruction when compared with normal individuals. The ROC curve analysis result showed that the best cut-off value to classify a person has PFT obstruction based on serum periostin is 24.556ng/ml or more. The comparison data of other biomarkers were provided in [Table/Fig-4].
Comparison of mean serum periostin and AEC between normal and obstructed PFT.
| Bood markers | PFT | Mean (SD) | t-value | p-value |
|---|
| Sr. Periostin(ng/ml) | Normal | 23.47 (3.046) | 9.301 | <0.001 |
| Obstructed | 45.20 (19.119) |
| AEC(cells/cu.mm) | Normal | 214.40 (96.441) | 5.260 | <0.001 |
| Obstructed | 751.89 (848.201) |
Sr. Periostin = Serum Periostin, AEC = Absolute Eosinophil counts, PFT = Pulmonary Function Test
ROC curve analysis of all the biomarker.
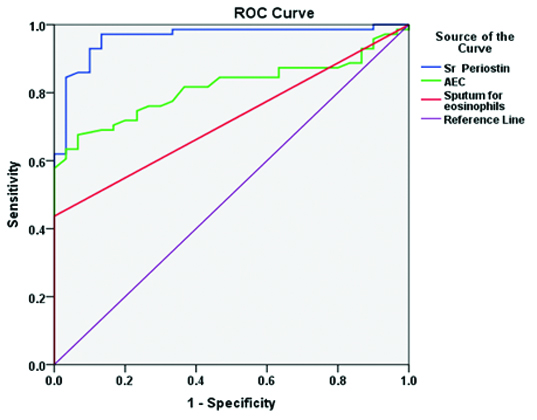
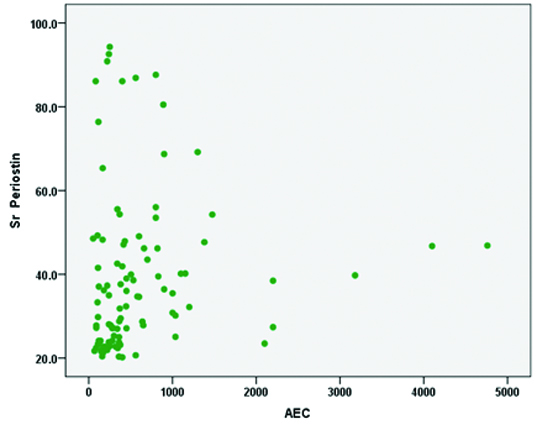
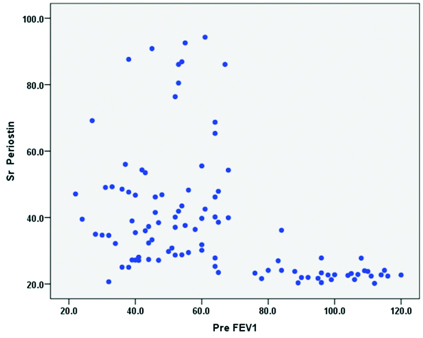
In the present study, the sensitivity of 97.18% made the biomarker periostin as an ideal non-invasive tool for diagnosis of Th2 high eosinophilic phenotypes asthma. The sensitivity and specificity analysis for various biomarkers are shown in [Table/Fig-5]. The Pearson correlation co-efficient test was performed to study the correlation between serum periostin and AEC levels. There is no positive correlation of serum periostin with AEC levels and the p-value is not significant [Table/Fig-6]. There is a positive correlation with the severity of obstruction, as the FEV1 value reduces there is a significant increase in serum periostin levels [Table/Fig-7].
Sensitivity, specificity analysis for serum periostin, AEC levels and sputum eosinophils.
| Parameter | Bood markers | Estimate | 95% CI (Lower - Upper) |
|---|
| Sensitivity | Sr. Periostin | 97.18% | (90.30, 99.22) |
| AEC | 67.61% | (56.06, 77.34) |
| Sputum eosinophils | 43.66% | (32.75, 55.23) |
| Specificity | Sr. Periostin | 86.67% | (70.32, 94.69) |
| AEC | 93.33% | (78.68, 98.15) |
| Sputum eosinophils | 100.0% | (88.65, 100.0) |
| Positive Predictive Value | Sr. Periostin | 94.52% | (86.74, 97.85) |
| AEC | 96.00% | (86.54, 98.90) |
| Sputum eosinophils | 100.0% | (88.97, 100.0) |
| Negative Predictive Value | Sr. Periostin | 92.86% | (77.35, 98.02) |
| AEC | 54.90% | (41.38, 67.73) |
| Sputum eosinophils | 42.86% | (31.94, 54.52) |
| Diagnostic Accuracy | Sr. Periostin | 94.06% | (87.64, 97.25) |
| AEC | 75.25% | (66.01, 82.64) |
| Sputum eosinophils | 60.40% | (50.65, 69.38) |
Sr. Periostin = Serum Periostin, AEC = Absolute Eosinophil counts
Correlation of serum periostin with AEC levels.
Scatter plot for correlation between serum periostin and pre FEV1.
Discussion
Asthma is regarded as an allergic disease mediated through Th2 driven inflammation. However, there is an emerging evidence of heterogeneity in the pathophysiology of asthma. Woodruff et al., showed that, mild to moderate bronchial asthma patients not on inhaled steroids, only about half of them had evidence of T-helper cell mediated inflammation of their airways [8]. Increased markers of allergy, bronchial fibrosis, eosinophilic airway inflammation and sensitivity to Inhaled Corticosteroids (ICSs) are the indications of highsubset in Th2 [7].
For the treatment of asthma, antagonists of Th2 cytokines IL-4, IL-5 and IL-13 are under investigation, which implies that it is necessary to identify the asthmatic patients most likely to benefit from these targeted therapies. Inflammatory pathways in the airways can be enabled directly through induced sputum sampling, measurement of exhaled gases and bronchoscopy [9,10]. However, they are expensive, time consuming, invasive and also they are not widely available in primary care settings. Thus, measurement of non-invasive biomarkers are beneficial to identify Th2-driven eosinophilic airway inflammation for targeted therapies.
Periostin, a protein from an extracellular matrix contributes to sub-epithelial thickening in bronchial airways and thereby it reflects the airway inflammation [11]. The deposition of periostin in the airway sub-epithelial layer in asthmatic patients was demonstrated by Takayama et al., [12].
Serum periostin has been identified as the single best predictor of airway eosinophilia in patients with severe asthma in the Bronchoscopic Exploratory Research Study of Biomarkers in Corticosteroid refractory Asthma (BOBCAT) [13]. Thomson et al., performed a double-blind, placebo-controlled study of lebrikizumab in 219 adults with uncontrolled asthma despite treatment with ICS [14]. Lebrikizumab treatment was effective in improving lung function in patients with high levels of serum periostin, which correlates with our present study [15]. Thus, identification of the serum periostin helps in managing the Th2 high eosinophilic phenotype as they do not respond to the traditional first-line therapy, i.e. ICS with Long Acting β-Agonists (LABA) combination and those patients might require anti-IL therapy for their improvement of chest symptoms [9,16].
The specificity of serum periostin is low, since it may be elevated in other conditions such as atopic dermatitis, IgG4-related sclerosing sialadenitis, eosinophilic otitis media, chronic rhinosinusitis, idiopathic pulmonary fibrosis, drug-induced pulmonary fibrosis, scleroderma, proliferative diabetic retinopathy, bone marrow fibrosis etc., [4,8,12]. In BOBCAT study, serum periostin levels correlated well with airway eosinophilia [13,17].
In the present study, ROC curve analysis showed the best cut-off value for serum periostin and the significant sensitivity and specificity values. This observation is in accordance with the BOBCAT study where the serum periostin cut-off value taken to be as 25 ng/ml, and the sensitivity and specificity was 95% and 83%, with a positive predictive value of 95% [13].
The results of the present study with respect to sensitivity and specificity of blood AEC levels were in accordance with Hastie et al., in which the authors reported 72% sensitivity and 69% specificity for the blood eosinophil count and also weak values for accuracy for sputum eosinophilia (range, 54% to 72%) with a sensitivity of 69% in ROC curve analyses [4]. Similar observations were made by Nair, where the author reported poor correlations between blood eosinophil and sputum eosinophil counts in patients with severe asthma and those with mild to moderate asthma [18]. However, in contrast, Pizzichini et al., stated that the proportions of eosinophils in sputum were a more accurate marker of asthmatic airway inflammation than the proportion of blood eosinophils [19].
Jia et al., have concluded in their study that the measurement of serum periostin level is the best predictor of airway eosinophilia among the other factors such as age, gender, body mass index, IgE levels and blood eosinophil [13]. In the present study, serum periostin had a positive correlation with sputum eosinophilia, where the sputum negativity for eosinophils/neutrophil predominant subgroups did not respond to ICS therapy. There was a negative correlation of blood AEC levels with that of serum periostin, it could be, due to treatment with ICS/LABA and oral steroid usage, which alters the blood AEC levels producing a negative correlation with that of serum periostin. The present study also showed a positive correlation with the severity of obstruction, as the pre FEV1 value reduced, there was a significant increase in serum periostin levels as depicted by a scatter plot graph with the results.
There are numerous therapies in clinical development targeting IL-13 and other factors driving T helper cell mediated inflammation in asthmatic patients. It is critical that these treatments are targeted to patients with relevant pathology because otherwise important treatment effects might be underestimated; studies of anti IL-5 therapy also highlights this point. The phase II studies of lebrikizumab, a humanized monocolnal antibody against IL-13, those asthmatic patients with high pretreatment serum periostin levels experienced substantially greater treatment benefit from IL-13 blockade than patients with low pre treatment periostin levels [12,15].
Limitations
The limitations of this study include limited sample size and patients were on varied doses of ICS/LABA which had a poor correlation with AEC levels and PFT with that of periostin levels.
Conclusion
The present report has shown that serum periostin is an ideal blood biomarker of Th2 high asthma and serum periostin had a higher sensitivity when compared to blood AEC and sputum eosinophils. Asthma patients who do not respond to ICS, the periostin levels should be measured. If it is high, Th2 antagonists should be considered for better improvement. There were very few reports on biomarker studies on bronchial asthma. Measurement of serum periostin in the diagnosis of Th2 high eosinophilic phenotype will be one of the landmark studies having a higher sensitivity and specificity rates, which is to be used for emerging asthma therapeutics targeting Th2 inflammation in the future.
Sr. Periostin = Serum Periostin, FEV1= Forced Expiratory Volume in one second, AEC = Absolute Eosinophil counts
Sr. Periostin = Serum Periostin, AEC = Absolute Eosinophil counts, PFT = Pulmonary Function Test
Sr. Periostin = Serum Periostin, AEC = Absolute Eosinophil counts