Aggressive periodontitis, is a unique type of periodontitis comprising of rapid destruction of periodontal ligament and alveolar bone in systemically healthy individuals generally of a younger age group but patients may be older [1,2] Its prevalence is less than chronic periodontitis, and result in early tooth loss in the affected individuals [3]. Aggregatibacter actinomycetemcomitans is frequently detected in patients with localized aggressive periodontitis. In generalized forms, a different microbiota like Porphyromonas gingivalis and Tannerella forsythus (formerly Bacteroides forsythus) have been isolated [4,5]. The most important goal of therapy is to reduce or eliminate these subgingival microorganisms, regenerate the lost tissues and maintain periodontal health. Scaling and root planing is considered as a gold standard to attain and maintain periodontal health by elimination of bacterial plaque. Although mechanical treatment significantly decreases the prevalence and levels of subgingival microorganisms, it does not necessarily eliminate all pathogens [6–8]. As the probing depth increases, the effectiveness of scaling and root planing decreases, leaving subgingival plaque, calculus on root surfaces and presence of persistent periodontopathogens [9,10]. A more efficient and atraumatic technique is the use of lasers for periodontal treatment [11]. Photodynamic therapy is a technique combining laser energy with a photosensitizer to produce singlet Oxygen molecules and free radicals to destroy targeted cells [12]. Both these therapies serve as a non-invasive approach for infection control [13] and has been used as adjuncts to mechanical therapy. This novel therapeutic approach seems promising in the non surgical treatment of aggressive periodontitis. Even though both PDT and SRP have been shown to have similar clinical results in the non surgical treatment of aggressive periodontitis [14], photodynamic therapy has advantages of reducing the treatment time and need for anaesthesia causing destruction of bacteria in a very short period of time without development of resistance by the target bacteria, and damage to adjacent host tissues. Periodontopathogens like Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis can infiltrate through the epithelial barrier into the periodontal tissues making their removal difficult in non-surgical periodontal therapy; elimination of these is therefore possible by photodynamic therapy [15]. We conducted a previous study using curcumin as photosensitizer in chronic periodontitis and noticed that curcumin used photodynamic therapy was a valuable method in treating chronic periodontitits [16]. Hence, the aim of the present prospective, controlled, clinical study is to evaluate clinically and microbiologically the effectiveness of the adjunctive use of photodynamic therapy with scaling and root planing versus lasers in non-surgical periodontal treatment in patients with aggressive periodontitis.
Materials and Methods
This clinical and microbiologic study was carried out in the Department of Periodontics, Coorg Institute of Dental Sciences, Virajpet. The study protocol was reviewed and approved by the Institutional Review board of Coorg Institute of Dental Sciences. The nature and purpose of the study and the recall protocol was explained to the subjects and a written consent was obtained before commencing the study. The sampling and method of collection of data was similar to previuos study done by same authors [16]. Inclusion Criteria for the study were patients who were diagnosed with either localized or generalized aggressive periodontitis aged between 18-35 years with at least one tooth with probing pocket depth ≥5 mm in each quadrant. Patients with periodontal treatment within the last 6 months, pregnancy, smoking, allergy to the dyes, systemic diseases, use of mouth rinses that could influence the outcome of therapy and ingestion of systemic antibiotics within the last 6 months were excluded from the study.
Out of 700 patients screened, 15 patients who fulfilled the above criteria were included in the study. Sixty sites among these 15 subjects were chosen for the study. All the treatment procedures were performed by the same operator in-order to prevent inter-operator variations. Scaling and root planing (SRP), diode laser irradiation and photodynamic therapy (PDT) was done on single patient in the same day on randomly chosen quadrants Q1, Q2, Q3, Q4 by flipping a coin. In addition the quadrant Q4 received PDT on multiple appointments. The analysis and recording of clinical parameters and microbiologic findings were performed by other author who was blinded to the treatment techniques and to the test and control sites in order to assure an unbiased determination (IS). The patients were followed up for a period of 3 months.
Collection of data
Upon screening and selecting the patients for study the following clinical examinations were done pre and postoperatively at 1st and 3rd months. Plaque Index (Sillness and Loe [17]) were recorded at baseline, 1st and 3rd month to monitor the oral hygiene and motivation of the patients. Sulcus Bleeding Index (Muhlemann & Son [18]), Probing pocket depth (PPD) and Relative Attachment Level (RAL) measured as distance between base of the pocket to apical extent of the occlusal stent (measured by using UNC-15 probe, Hu-friedy, USA.) were taken at baseline which was repeated after 3 months.
Treatment Protocol
A proforma was used consisting of a brief case history, clinical examination and recordings of clinical parameters at baseline, 1 month and at the end of 3 months. Oral hygiene instructions for supra gingival plaque control were given. Each quadrant was randomly assigned to one of the four treatment modality.
Quadrant 1 (Q1): Scaling and root planing (SRP) alone with ultrasonic scaler. {Satelec P5® SATELEC (India) PVT. Ltd. Gandhinagar, India}.
Quadrant 2 (Q2): SRP + Laser irradiation with 810 nm at 1W, continuous mode for 30 Seconds per tooth. {AMD Picasso® DENTSPLY India Pvt. Ltd, India}.
Quadrant 3 (Q3): SRP + Photodynamic Therapy (PDT) on “0” day {Toluidine blue – O dye 1mg/ml and diode laser 810 nm}.
Quadrant 4 (Q4): SRP + PDT on 0, 7th and 21st day {Toluidine blue – O dye 1mg/ml and diode laser 810 nm}.
Plaque sampling for microbial analysis: After meticulous removal of supra-gingival plaque and calculus, the area was dried and isolated with cotton rolls saliva evacuators then sub-gingival plaque samples were collected using sterile universal curettes (Hu-Friedy, USA.) from each selected site (deepest pocket in each quadrant). Samples were collected at baseline, 1 month and at 3 months postoperatively. Samples were placed in sterile vials containing 0.5ml of the Reduced Transport fluid (RTF) and sent to the laboratory for microbial analysis.
Procedure for photodynamic therapy (PDT): PDT was done with a diode laser 810nm at 0.1W power output, continuous mode with toluidine blue-O 1mg/ml solution as a photo sensitizer in quadrants 3 and 4 [19]. The photo sensitizer was applied to the bottom of the periodontal pocket with the help of an applicator. After 3 minutes of action the photo sensitizer was rinsed with saline and exposed to diode laser on “0” day which was repeated on 7th and 21st day for site in quadrant 4. The procedure was done using standard laser safety protocol including protective eye wear for the operator, patient and the assistant. The patients were then instructed for routine brushing and rinsing with water. The patients were informed about the complications like feeling of pressure, pain or irritation in the area and were advised to have soft diet. Only if the irritation became intolerable the patients were advised to report back to the department.
Bacterial Culturing: The microbial samples were assessed for Aggregatibacter actinomycetemcomitans and black pigmented bacteroids (BPBs); Porphyromonas gingivalis & Prevotella intermedia by anaerobic bacterial culture method.
For Aggregatibacter actinomycetemcomitans (TSBV) media [20] was prepared with tryptic soy agar into which was added 10% serum, 1mg/ml yeast extracts, 75 μg/ml Bacitracin and 5 μg/ml Vancomycin.
For black pigmented bacteroids (BPBs) media [21] was prepared with blood agar base with 5% rabbit blood, 5μg/ml Haemin, 0.5μg/ml Menadione, 40μg/ml Kanamycin.
The constituents were dispensed as per composition, weighed on an electronic balance and added to the required volume of distilled water. Upon sterilization and returning to room temperature the previously weighed quantities of antibiotics and blood were mixed to the respective solutions. The media was quickly poured in petri plates under strict sterile conditions in the laminar air flow chamber and allowed to cool. The samples were dispersed by ultrasonication for 1min in a vortex mixture. Portion of these samples taken in an inoculation loop of diameter 2mm were plated, in triplicate, on plates containing BPB media and TSBV.
All plates were incubated at 37°C for 72 hours in an atmosphere of 5% CO2 in an anaerobic jar. After completion of incubation the plates were removed and microorganisms were confirmed by colony morphology and the colony characteristics of the respective organism. The colony count was done in a manual counter for quantification.
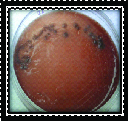
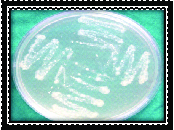
Black pigmented bacteroids (Porphyromonas gingivalis & Prevotella intermedia) were identified as black pigmented colonies with β-haemolysis on blood agar [Table/Fig-1]. Aggregatibacter actinomycetemcomitans (Aa) was identified as minute, white translucent colonies on TSBV agar [Table/Fig-2].
Microbial sample culture for Black pigmented bacteria.
Microbial sample culture for Aa
Results
A total of 15 patients who were diagnosed with aggressive periodontitis consisting of 9 females of mean age 27.33±2.29 and 6 males with mean age 27.83 ±3.71 were included in the study. Quantitative analysis was done for all the clinical and microbiologic parameters by calculating mean and standard deviation. Collective data was analysed by Kruskall Wallis ANOVA. Chi-square and Z-test were used for bleeding on probing, probing pocket depth and clinical attachment level and microbial CFU at baseline and postoperatively within the group. To compare the effect between the groups Mann Whitney test was used. P-value < 0.05 is considered to be statistically significant. Data was analysed using statistical software SPSS (Statistical Package for Social Science, Version 13.0 in Microsoft Excel).
When compared within the groups all the clinical parameters showed a statistically significant difference in reduction of mean values from baseline to 1 month and from 1 month to 3 months with p-value < 0.05 [Table/Fig-3] which shows the effectiveness of individual therapeutic modalities. Statistically significant reduction in clinical parameters like PI, BOP, PPD and RAL was observed for each treatment modality. The gain in RAL from sites Q1 to sites Q4 was observed to be 0.28>0.46>0.65>0.76 respectively. [Table/Fig-3]. Similar concomitant decrease in the microbial CFU from sites Q1 through sites Q4 with mean differences of 20.34>40.2>51.13>55.33 was observed for Aa as well as for BPB 28.47>35.06>44.67>47.87 respectively. This shows the increasing benefit of simple laser irradiation adjunct to scaling and root planing and even more effectiveness of PDT over SRP and Laser irradiation [Table/Fig-4,5].
Intra group comparison – Clinical parameters.
| Quadrants | | Plaque index | BOP | PPD | RAL |
|---|
| | Baseline | 3rd month | Baseline | 3rd month | Baseline | 3rd month | Baseline | 3rd month |
|---|
| I | Mean values | 2.61±0.18 | 1.58±0.26 | 3.16±0.35 | 1.72±0.20 | 6.08±0.21 | 5.79±0.20 | 9.41±0.51 | 9.13±0.46 |
| Difference | 1.03# | 1.44# | 0.29# | 0.28# |
| 2 | Mean values | 2.51±0.20 | 1.59±0.25 | 3.28±0.35 | 1.58±0.23 | 6.13±0.35 | 5.63±0.47 | 9.63±0.77 | 9.17±0.84 |
| Difference | 0.92# | 1.7# | 0.5# | 0.46# |
| 3 | Mean values | 2.53±0.20 | 1.54±0.28 | 3.27±0.34 | 1.57±0.23 | 6.20±0.25 | 5.59±0.53 | 9.52±0.58 | 8.87±0.71 |
| Difference | 0.99# | 1.7# | 0.61# | 0.65# |
| 4 | Mean values | 2.54±0.21 | 1.51±0.29 | 3.27±0.32 | 1.39±0.17 | 6.21±0.27 | 5.40±0.47 | 9.93±0.43 | 8.63±0.61 |
| Difference | 1.03# | 1.88# | 0.8# | 0.76# |
#Differences statistically significant with p-value<0.05
Intra group comparison – Microbial parameters.
| Quadrants | | Aa | BPB |
|---|
| | Baseline | 1st month | 3rd month | Baseline | 1st month | 3rd month |
|---|
| I | Mean values | 143.07±14.85 | 123.60±12.09 | 122.73±8.52 | 132.67±15.81 | 110.93±13.49 | 104.20±12.81 |
| Difference | Baseline to 1st month1st month to 3rd monthBaseline to 3rd month | 19.470.87#20.34# | 21.74#6.73#28.47# |
| 2 | Mean Values | 142.87±10.78 | 116.27±8.50 | 102.67±10.50 | 128.13±10.89 | 105.73±7.70 | 93.07±5.85 |
| Difference | Baseline to 1st month1st month to 3rd monthBaseline to 3rd month | 26.6#13.6#40.2# | 22.4#12.66#35.06# |
| 3 | Mean Values | 144.53±11.82 | 115.87±9.12 | 93.40±8.79 | 133.27±11.48 | 106.93±8.79 | 88.60±7.73 |
| Difference | Baseline to 1st month1st month to 3rd monthBaseline to 3rd month | 28.66#22.47#51.13# | 26.34#18.33#44.67# |
| 4 | Mean Values | 136.00±11.63 | 105.13±10.02 | 80.67±9.58 | 127.27±11.82 | 99.93±10.38 | 79.40±10.34 |
| Difference | Baseline to 1st month1st month to 3rd monthBaseline to 3rd month | 30.87#24.46#55.33# | 27.34#20.53#47.87# |
# Differences statistically significant with p-value <0.05
| Parameters | Intervals | Site 1 | Site 2 | Site 3 | Site 4 | Diff. b/n 1&2 | Diff. b/n 2&3 | Diff. b/n 3&4 |
|---|
| PI | Baseline | 2.61±0.18 | 2.51±0.20 | 2.53±0.20 | 2.54±0.21 | 0.10 | 0.02 | 0.01 |
| 1 month | 2.35±0.21 | 2.27±0.33 | 2.28±0.23 | 2.24±0.27 | 0.08 | 0.01 | 0.04 |
| 3 months | 1.58±0.26 | 1.59±0.25 | 1.54±0.28 | 1.51±0.29 | 0.01 | 0.05 | 0.03 |
| BI | Baseline | 3.16±0.35 | 3.28±0.35 | 3.27±0.34 | 3.27±0.32 | 0.12 | 0.01 | 0.00 |
| 3 months | 1.72±0.20 | 1.58±0.23 | 1.57±0.23 | 1.39±0.17 | 0.14 | 0.01 | 0.18# |
| PPD | Baseline | 6.08±0.21 | 6.13±0.35 | 6.20±0.25 | 6.21±0.27 | 0.05 | 0.07 | 0.01 |
| 3 months | 5.79±0.20 | 5.63±0.47 | 5.59±0.53 | 5.40±0.47 | 0.34 | 0.04 | 0.19 |
| RAL | Baseline | 9.41±0.51 | 9.63±0.77 | 9.52±0.58 | 9.39±0.43 | 0.22 | 0.11 | 0.13 |
| 3 months | 9.13±0.46 | 9.17±0.84 | 8.87±0.71 | 8.63±0.61 | 0.04 | 0.30 | 0.24 |
| Aa | Baseline | 143.07±14.85 | 142.87±10.78 | 144.53±11.82 | 136.00±11.63 | 0.20 | 1.66 | 8.53# |
| 1 month | 123.60±12.09 | 116.27±8.50 | 115.87±9.12 | 105.13±10.02 | 7.33 | 0.40 | 10.74# |
| 3 months | 122.73±8.52 | 102.67±10.50 | 93.40±8.79 | 80.67±9.58 | 20.06# | 9.27# | 12.73# |
| BPB | Baseline | 132.67±15.81 | 128.13±10.89 | 133.27±11.48 | 127.27±11.82 | 4.54 | 5.14 | 6.00 |
| 1 month | 110.93±13.49 | 105.73±7.70 | 106.93±8.79 | 99.93±10.38 | 5.20 | 1.20 | 7.00# |
| 3 months | 104.20±12.81 | 93.07±5.85 | 88.60±7.73 | 79.40±10.34 | 11.13# | 4.47 | 9.20# |
# Statistically significant difference with p-value < 0.05
Intergroup comparison between all quadrants had comparable mean values at baseline with respect to PI, PPD and RAL with statistically non-significant values (p-value>0.05) indicating an unbiased distribution of quadrants for different treatment interventions. Between quadrant 1(Q1) and quadrant 2 (Q2) comparison with respect to the microbial parameters showed a comparable reduction in CFU of Aa and BPB in both the sites after 1 month. But at 3 months postoperatively, the sites from quadrant 2 showed better reductions compared to sites from quadrant 1 with statitistically highly significant difference with p-value 0.000. Similarly, For BPB at 3 months a statistically significant reduction with p-value 0.032 was observed indicating the added benefits in use of laser as an adjunctive to SRP [Table/Fig-4].
On comparison between the sites from Q2 and Q3 with respect to the microbial parameters showed similar reduction in CFU of Aa in both the sites at 1 month and a statistically significant more reduction in sites from Q3 was noticed at 3 months with p-value 0.018 [Table/Fig-5] indicating single application of PDT had better effect over simple LASER irradiation as an adjunct to SRP. Similarly for Black pigmented bacteria (BPB) the sites from both the quadrants showed similar comparable reductions in CFUs at 1 and 3 months. The difference in reduction was statistically not significant with p-values 0.547 and 0.270 respectively [Table/Fig-5].
When compared between the quadrant of single application of PDT (Q3) and multiple application of PDT (Q4), all the clinical parameters showed similar reduction in values at 3 months postoperatively except, bleeding on probing in which Q4 showed better reduction compared to Q3 at 3 months with p-value 0.012 which was further reflected in reduction of CFUs of Aa and BPB at 3 months with p-value 0.003 and 0.011 respectively. It can now be directly attributed to the beneficial effects multiple application of PDT over single application [Table/Fig-5].
Discussion
The treatment of aggressive periodontitis is challenging for clinicians as there are no established protocols and guidelines for efficient control of the disease [22]. The treatment measures include conventional mechanical nonsurgical and surgical treatments with various adjunctive anti-infective therapies with disinfectants and antibiotics [23]. Disadvantages of chemotherapeutic treatment are possible bacterial resistances and side effects following systemic administration [24]. Photodynamic therapy obviates these disadvantages by combining soft laser irradiation with photosensitizer dye. It has adjunctive benefit at sites with deep periodontal pockets, furcations and root concavities.
In order to explore the effectiveness of photodynamic therapy in destroying periodontal bacteria in vivo the present prospective controlled split mouth study was carried out using 810nm diode laser as an adjunct to scaling and root planing. Toluidine blue was chosen as the photosensitizer in this study, because it has been shown to be potentially one of the safest photosensitizers for treating periodontal disease [25–27].
When compared within each group all the clinical parameters showed a significant reduction in their values between baseline to 3 months suggesting the effectiveness of individual therapy. Scaling and root planing (SRP) is one of the most commonly utilized clinically effective procedure for the treatment of periodontal disease regarded as the gold standard treatment which has been supported by various studies [28–35]. The clinical benefits are derived from the removal of subgingival plaque and disruption of subgingival biofilm leading to a decrease of bacterial counts. In the present study, SRP alone (Sites-1) was capable of appreciably decreasing the counts of the bacterial species tested, namely, A. actinomycetemcomitans (Aa) and black pigmented bacteria (BPB) consisting of P. gingivalis, and P. intermedia. These data confirm the favourable effect of SRP in decreasing the bacterial levels.
Lasers have been introduced as an adjunctive tool to mechanical therapy. They have the potential of a bactericidal and detoxification effect. In our study the laser-assisted treatment (Sites-2) showed lower bacterial levels as compared to SRP alone (Sites-1) at 3 months postoperatively. This favourable effect might be due to the ability of laser irradiation to eliminate bacteria from the periodontal pockets as confirmed from previous studies [36–38].
A greater improvement in clinical & microbiological parameters have been observed in our study with the application of lasers along with Toluidine-O-blue dye in photodynamic therapy when used as an adjunct to SRP (Sites-3). These results are in accordance with previous studies [39–41]. In the present study, the mean microbial levels decreased significantly in the all the groups [Table/Fig-4]. The sites which received PDT showed greater efficiency in reducing the bacterial counts. i.e., at 3 months follow up, the bacterial count for Aa reduced to 93.40 ±8.79 from 144.53±11.82 at baseline and for BPB reduced to 88.60±7.73 from 133.27±11.48 in single application of PDT sites (Sites-3, [Table/Fig-4]). Similarly, in multiple application of PDT on 0, 7th and 21st day the bacterial counts reduced to 80.67±9.58 from 136±11.63 at baseline and for BPB reduced to 79.40±10.34 from 127.27±11.82 showing a better reduction in (sites-4) values compared to all other sites. These results were statistically highly significant with p-value of 0.001 [Table/Fig-4]. These results were in accordance with similar other studies being conducted on the same bacteria wherein combination of photosensitizer with lower level laser therapy was effective in killing Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, and Fusobacterium nucleatum [42].
Limitations
This is a pilot study undertaken with only15 patients with aggressive periodontitis to evaluate the efficacy of photodynamic therapy on Aggressive periodontitis using Diode laser and Toluidine blue as photosensitizer as an adjunct to SRP. In this study even though all the sites showed statistically significant reduction in bacterial counts from baseline to 3 months, all the sites still had significant number of viable bacteria. Even sites with multiple application of PDT had mean residual bacterial counts of 80.67 for Aa and 79.40 for BPB at 3 month postoperatively. This may be attributed to the use of toluidine blue ‘O’ dye having a peak absorption wavelength in the range of 620 – 700 nm [43]. The diode laser used in this study was of 810 nm which might be the reason for incomplete effectiveness of PDT over SRP and laser irradiation. Moreover, the bacteria which are considered in this study was Aa and other black pigmented bacteria like Pg and Pi which are shown to invade the periodontal soft tissues in previous studies [44]. Therefore these connective tissue invaded bacteria can act as reservoir which can multiply and recolonise the subgingival plaque samples.
Conclusion
Within the limitations of the study it can be concluded that Diode laser irradiation with 810 nm wavelength and a power intensity of 1 Watt as an adjunctive to SRP has an antibacterial action on periodontal pathogens such as Aa, Pg and Pi. This provides a platform over which the effects of Photodynamic therapy using Toluidine Blue ‘O’ dye 1mg/ml and diode laser 810 nm with power output 0.1 Watt as an adjunct to SRP can be effectively compared to show its enhanced effects. Our study showed that the effects were further magnified by multiple applications of PDT. Therefore, further studies can be undertaken by taking connective tissue scrappings instead of subgingival plaque samples and selection of dye with penetrating ability in the periodontal ligament tissues with specific wavelength and bacteria of interest.
#Differences statistically significant with p-value<0.05
# Differences statistically significant with p-value <0.05
# Statistically significant difference with p-value < 0.05