The gastric mucosa can be divided into three histological areas like superficial, neck and deep zones. The superficial zone composed of single layer of mucin secreting columnar cells. The neck zone with largely of immature stem cells mixed with some mucous neck cells. The deep zone is composed of glands, the bases of which lie close to or in muscularis mucosae [1].
The epithelium of the human gastric mucosa produces different types of mucins. Mucins of the stomach are large glycoproteins with attached side chain of sugar residue, N acetyl derivative of hexosamine and terminal sialic acid group [2]. They perform significant functions like selective impermeability to hydrochloric acids and bile salts, antibacterial effects, lubricating the luminal contents, regulate epithelial dehydration, prevent tissue edema, anti-metastatic and immunological phenomenon [3]. Understanding the mechanisms involved in the biosynthesis and degradation mucins along with the analysis of genes coding for the mucins proteins may give insight into the factors that lead to the development of various pathological conditions of the stomach [3,4].
Any alteration in the production of mucins either their amounts or their biochemical composition are implicated in the aetiology of gastric intestinal metaplasia, gastric erosions or ulcerations [5–7]. Now-a-days pathologists have applied histochemical methods to detect altered mucin secretions to confirm the diagnosis and prognosis of the gastric pathology [8]. The present work has been focused at analysing the nature of mucins secreted by the epithelial cells in different parts of gastric mucosa and to highlight the functional significance of those mucins in health and illness.
Materials and Methods
Fifty stomach specimens were collected from the resected free margins of gastrectomy specimens, collected from August 2012–July 2015. Normal gastric mucosas 5 cm distal to the ulcerated and carcinomatous lesions were processed for the study. We discarded the specimens with previous history of chronic ingestion of drugs, patients with prolonged illness, poisoning specimens as they fail to produce good quality histological structures. The samples were taken from the different sites of the stomach like fundus, body and pylorus. The samples were fixed with 2% calcium acetate in 10% formaline and processed routinely. Two sections from each specimens were stained with haematoxylin and eosin method and the rest with various histochemical techniques like – Alcian Blue 8GX (AB) staining at pH 1.0 and pH 2.5 to identify the sulphated mucins; Periodic Acid Schiff (PAS) to identify the neutral mucins; Combined Alcian Blue and Periodic Acid Schiff (AB pH 2.5 – PAS) to show the difference in the amount of neutral and acidic (sulpho and sialo) mucins secretions; Aldehyde Fuschin (AF) to identify the sialomucins; Combined Aldehyde Fuschin and Alcian Blue (AF – AB pH 2.5) staining to differentiate the sulpho and sialomucins. The procedures for each of the above said staining as well as the solutions of AB at pH1 and 2.5 (Mowry, 1960), Schiff reagent for PAS (Mc Mannus, 1956), AF solution (Gomari, 1950) were prepared and the procedures were followed as per the textbook “Lynch’s medical laboratory technology” by Culling [9].
Results
The distribution of mucins in epithelial elements of adult stomach fundus, body and pylorus regions were noted as depicted in [Table/Fig-1,2 and 3] respectively.
Distribution of mucins in epithelial elements of adult stomach (fundic part)
| Sl. No. | Histo. Tech. | Sup. Zone | Neck zone | Deep zone |
|---|
| Surface epithelium | Foveolar cell | Mucous neck cell | Fundic gland |
|---|
| 1. | AB pH 1 | - ve | - ve | - ve | ± B |
| 2. | AB pH 2.5 | - ve | - ve | + B | + B |
| 3. | PAS | +++ M | ++M | ++ M | ±M |
| 4. | AB pH 2.5 – PAS | ++ M | +MP | + MP | ± B |
| 5. | AF | - ve | - ve | - ve | ± ve |
| 6. | AF – AB pH 2.5 | - ve | - ve | - ve | + B, ± PP |
M-Magenta, P-Purple, MP-Magenta-purple, B-Blue, BP-Blue-purple, PP-Pink-purple
- ve : Negative staining
± : Weak or variable staining
+ : Slight staining
++ : Moderate staining
+++ : Strong staining
Distribution of mucins in epithelial elements of adult stomach (body part)
| Sl. No. | Histo. Tech. | Sup. Zone | Neck zone | Deep zone |
|---|
| Surface epithelium | Foveolar cell | Mucous neck cell | Body gland |
|---|
| 1. | AB pH 1 | - ve | - ve | ± B | ± B |
| 2. | AB pH 2.5 | - ve | - ve | + B | + B |
| 3. | PAS | +++ M | ++ M | ++ M | ++ M |
| 4. | AB pH 2.5 – PAS | ++ MP | + MP | + MP | +± MP |
| 5. | AF | - ve | - ve | ± PP | + PP |
| 6. | AF – AB pH 2.5 | - ve | - ve | - ve | ± P, ++B |
M-Magenta, P-Purple, MP-Magenta-purple, B-Blue, BP-Blue-purple, PP-Pink-purple
- ve : Negative staining
± : Weak or variable staining
+ : Slight staining
++ : Moderate staining
+++ : Strong staining
Distribution of mucins in epithelial elements of adult stomach (pyloric part).
| Sl. No. | Histo. Tech. | Sup. Zone | Neck zone | Deep zone |
|---|
| Surface epithelium | Foveolar cell | Mucous neck cell | Pyloric gland |
|---|
| 1. | AB pH 1 | - ve | - ve | + B | + B |
| 2. | AB pH 2.5 | - ve | - ve | ± B | ± B |
| 3. | PAS | +++ M | ± M | + M | +++ M |
| 4. | AB pH 2.5 – PAS | ++ MP | ± MP | + MP | ++ MP |
| 5. | AF | - ve | - ve | - ve | + P P |
| 6. | AF – AB pH 2.5 | - ve | - ve | - ve | ± BP |
M-Magenta, P-Purple, MP-Magenta-purple, B-Blue, BP-Blue-purple, PP-Pink-purple
- ve : Negative staining
± : Weak or variable staining
+ : Slight staining
++ : Moderate staining
+++ : Strong staining
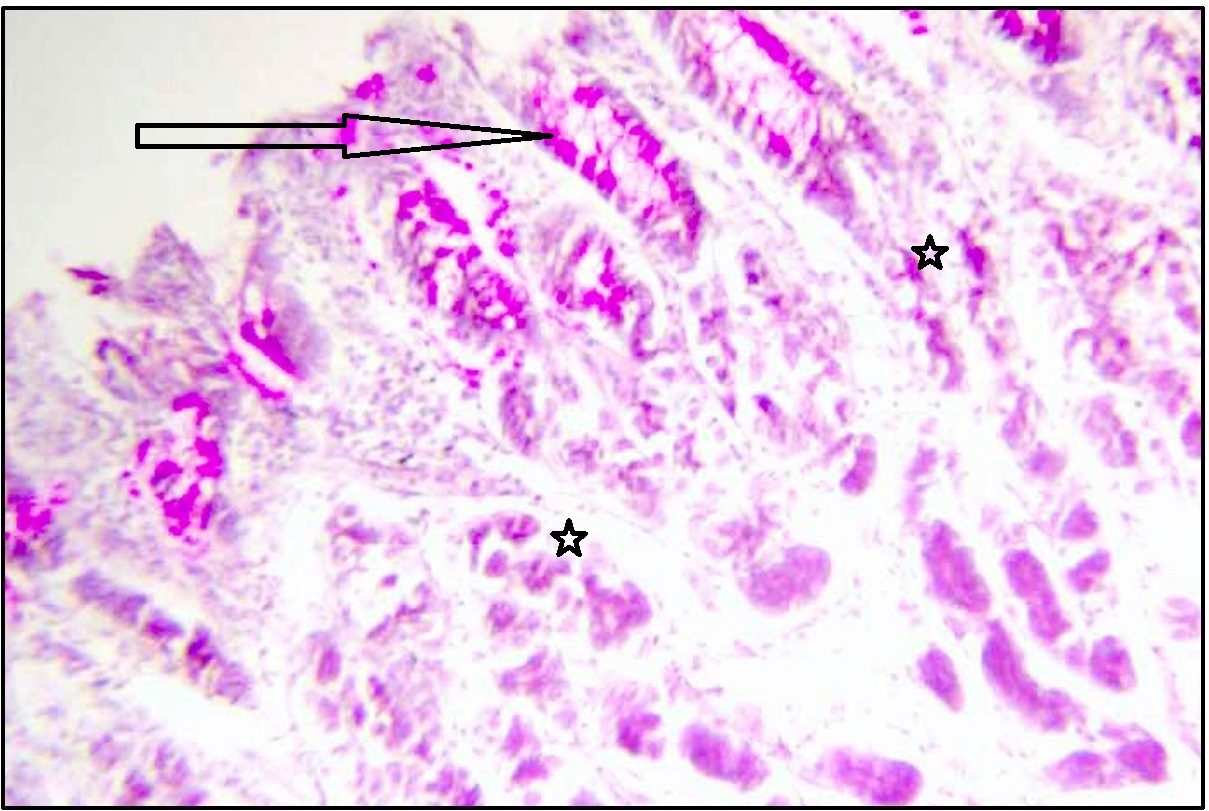
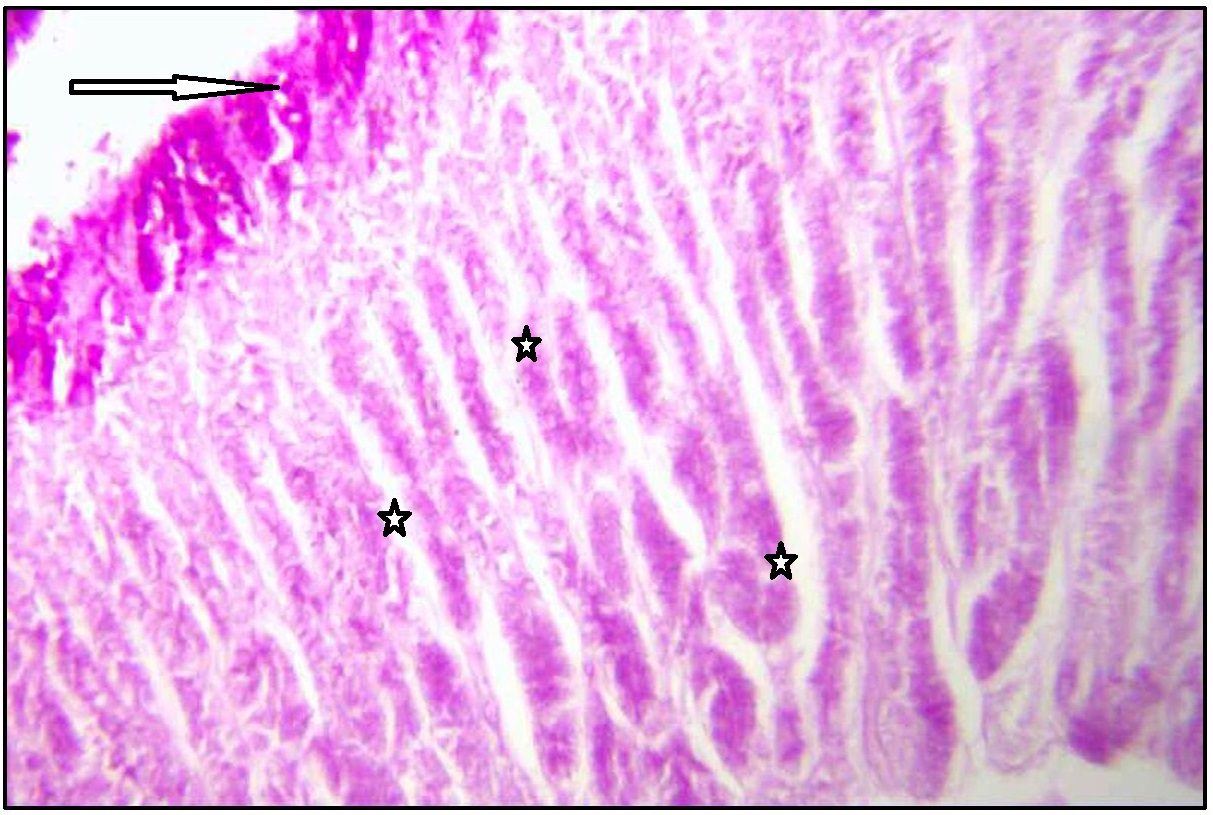
With PAS technique [Table/Fig-4,5], surface epithelium and foveolar cells of normal gastric mucosa showed strong magenta staining. But surface epithelium of fundic, body and pyloric parts showed negative reaction with AB pH 1 and pH 2.5. Trace amount of acid mucin was seen inconsistently in deep part of gland and deep foveolar cells.
Stomach (Fundus) showing neutral mucin in surface epithelium (arrow marked), foveolar cells, and weakly stained mucus neck cells (star marked). Stained with PAS. Magnification x 100.
Stomach (Body) showing neutral mucin in surface epithelium (arrow marked), foveolar cells (upper star), and weakly stained mucus neck cells (lower star). Stained with PAS. Magnification x 100.
Deep foveolar cells shoed weak alcinophilia in fundic, body and pyloric part of stomach. Mucous neck cells showed weak alcinophilia with AB pH 2.5.
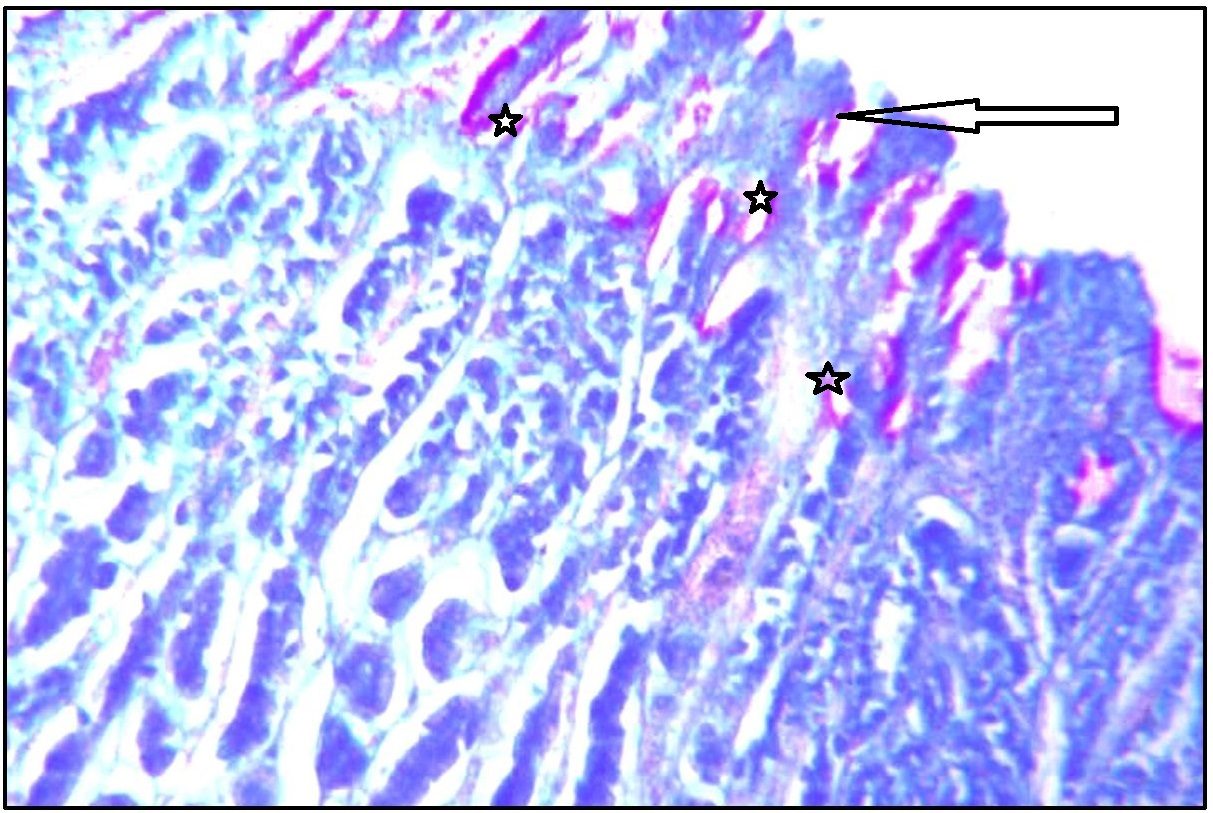
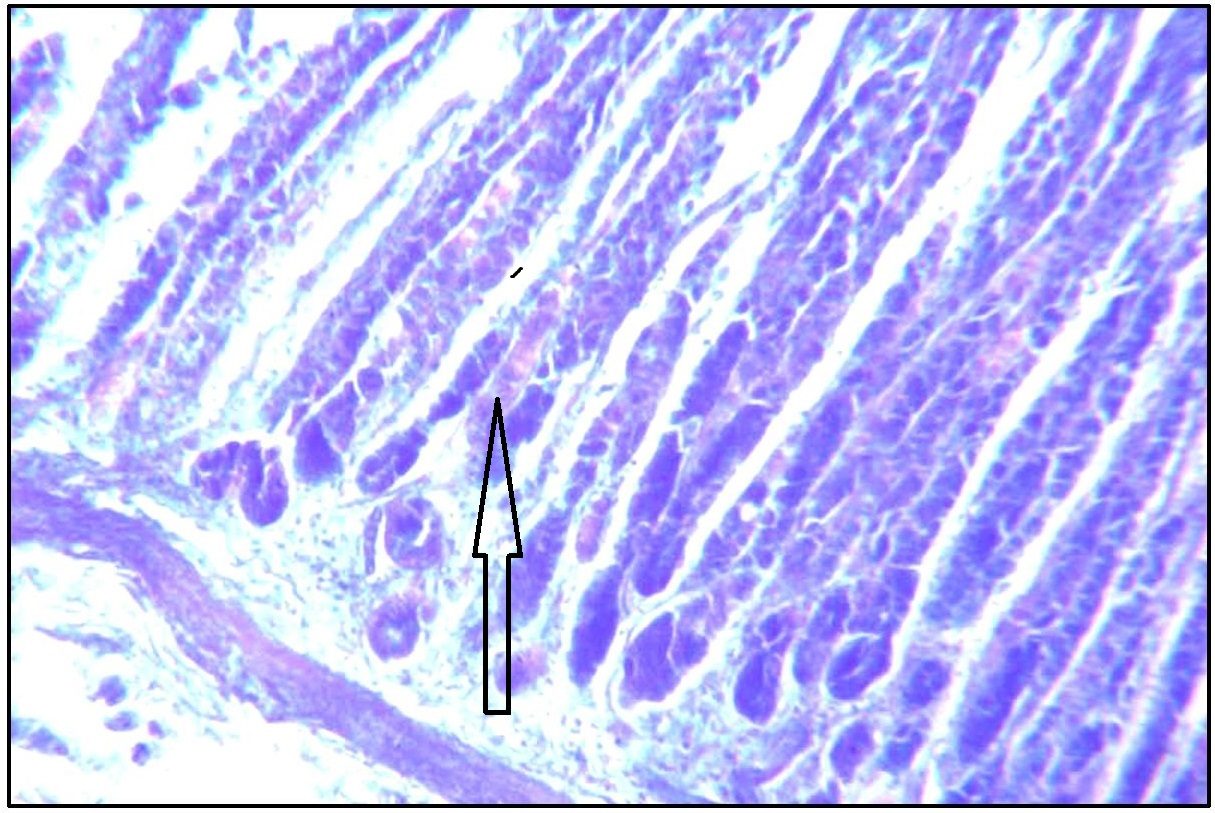
With AB pH 2.5 – PAS, surface epithelium of fundic part showed increased neutral mucin with intense magenta staining [Table/Fig-6]. Deep foveolar cells showed magenta to purple staining indicating presence of predominant neutral mucin with trace of acid mucin [Table/Fig-7].
Stomach (Fundus) showing neutral mucin in surface epithelium (arrow marked) and mucus neck cells (star marked). Stained with AB pH 2.5-PAS. Magnification x 100.
Stomach (Body) showing magenta purple stained body glands (arrow marked). Stained with AB pH 2.5-PAS. Magnification x 100.
With AF – AB pH 2.5, normal fundic stomach shows trace amount of sialomucin and sulphomucin [Table/Fig-8]. With AF technique, gastric mucosa showed negative reaction in fundic region. It was observed that gastric glands of fundic region showed trace amount neutral and acid mucin.
Stomach (Body) showing sialomucin and traces of sulphomucins (arrow marked). Stained with AF-AB pH2.5. Magnification x 400.
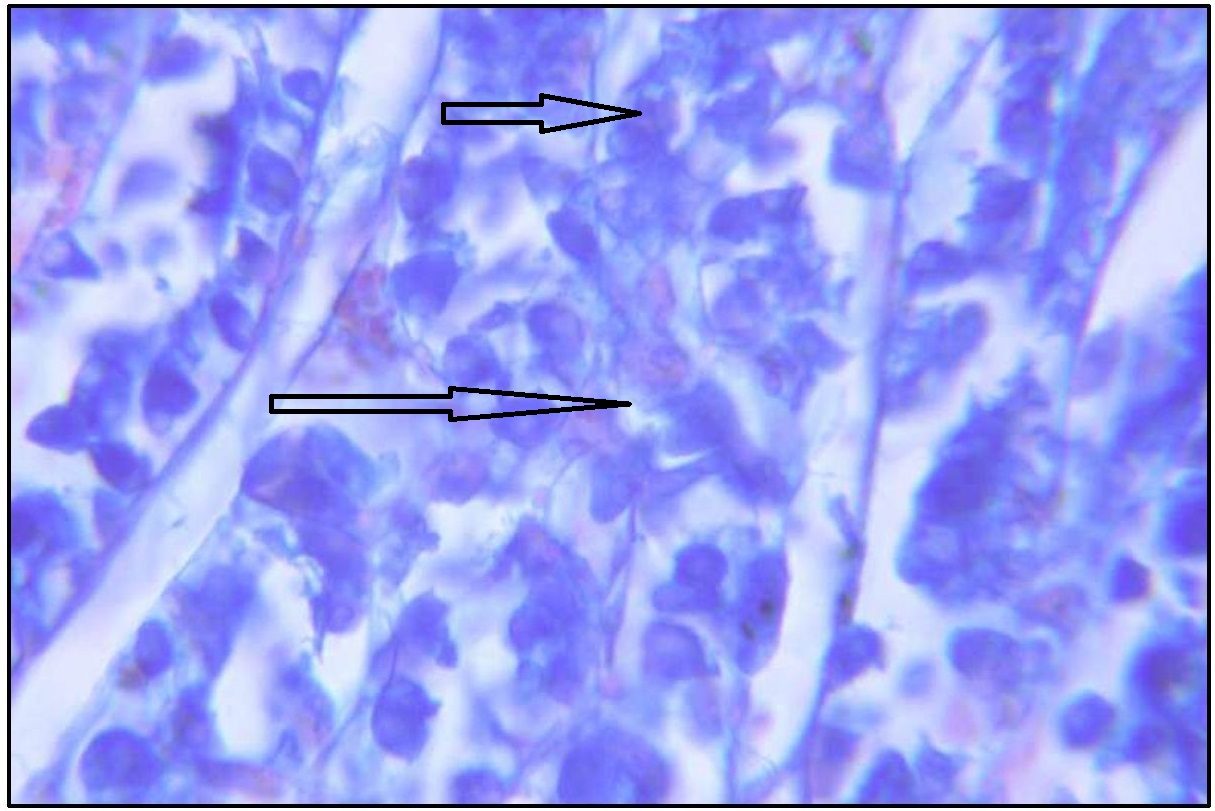
Foveolar cells showed neutral and acid mucin, which is confirmed by AB pH 2.5 – PAS. Mucous neck cells in neck zone of gastric mucosa showed magenta to purple staining. With AB pH 2.5 – PAS, pyloric glands showed magenta to purple staining showing predominant neutral mucin and trace amount of sialomucin.
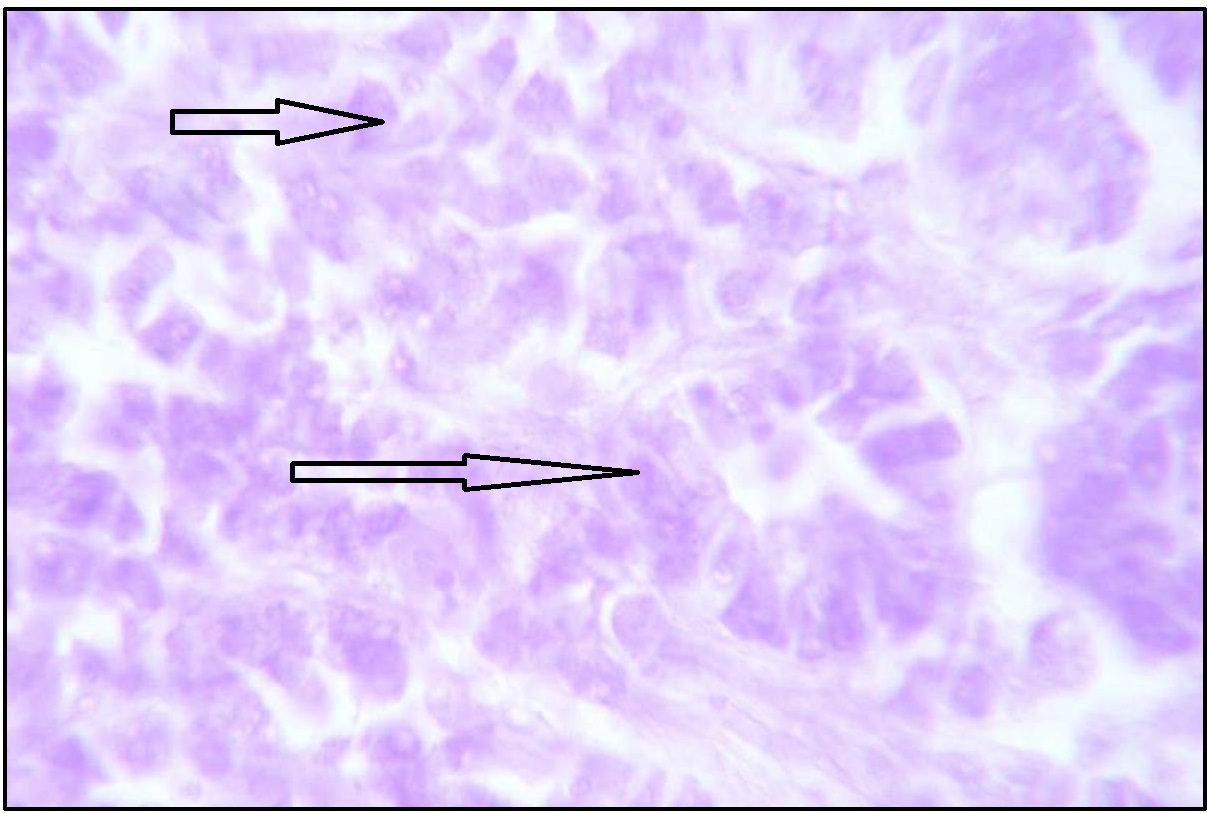
With AF – AB pH 2.5, pyloric glands showed negative staining. Trace amount of sialomucin was observed at the base of deep zone of pyloric part [Table/Fig-9]. With AF technique negative staining was observed indicating negative to variable amount of sulphomucin.
Stomach (Pylorus) showing traces of suplhomucin in the glandular region (arrow marked). Stained with AF. Magnification x400.
Discussion
Histochemically mucins are classified into two types: Epithelial mucin (mucins/mucosubstances) and Connective tissue mucin (mucopolysaccharides). Epithelial mucins are further classified as neutral and acidic. The latter further grouped as sialo- and sulphomucins depending on the sialic acid or ester sulphate radicals with hexosamine [10,11]. The present study is restricted to the epithelial mucins only.
Gad A showed the surface epithelium of the stomach together with foveolar cells, cardiac and gastric glands are the source of bulk of neutral mucin secreted by the stomach. The main origin of sialomucin is the mucous neck cells although the foveolar cells and surface epithelium contribute to it [3,8]. In the present study, surface epithelium shows PAS positive staining in supranuclear position indicating predominant neutral mucin.
The present study also demonstrates two types of glands: Zymogenic glands seen in the fundus and body and glands which contain PAS positive cells in the pyloric region. The transitional types of glands were described by Aschoff and Billenkamp [9]. This type of glands shows features transitional between principal and pyloric glands. In the present study this type of gland is not found in the human stomach mucosa.
In the present study the parietal cells are large polyhedral cells with eosinophilic granules in the cytoplasm and oval to round central nuclei, found in the principal gastric glands found in the fundus and body. PAS positive staining in parietal cell may be because of glycoprotein nature of intrinsic factors [8].
Bensely RR study reveals the principal gastric glands are lined by different types of cells. The predominant cell of the principal gastric glands is the zymogenic cell (or chief cell) [9]. It secretes pepsinogen. Bewnie showed a method to stain pepsinogen granules. These zymogemic cells are columnar; cytoplasm is basophilic and granular; the nucleus is round and basally placed [10].
Gad A reported that the gastric glands are the source of the bulk of neutral mucin secreted by the stomach. Sulphomucin can be demonstrated in deep foveolar and mucous neck cells [3,8].
The present work with AB pH 2.5 – PAS sequential staining indicates predominant neutral and small amount of acid mucin. With AF-AB pH 2.5 combined technique, trace amount of sulphomucin and small amount of sialomucin are seen in deep foveolar and mucous neck cells.
The present study clearly shows violet colour was more intense in basal and apical glandular areas. In this case, material which initially reacted only to PAS also revealed AB reactivity subsequent to use of the PAS/AB technique. The result was an intense violet colour.
The mucins secreted by the normal epithelial cells shows altered polarity in response to stress. With the loss of polarity, apical proteins, the transmembrane mucins are repositioned over the epithelial cell membrane. This lead to epithelial-mesenchymal transition and transform into cancer cells with stem cell-like characters and aggressive behaviour [3,5].
All types of mucosubstances - neutral, sialo and sulphomucins, are secreted in relatively increased amounts by the surface epithelium and the glands of the stomach of the fetus. Mucus neck cells and deep foveolar cells with increased section of neutral and acid mucin (sulphomucin and sialomucin) resemble with intermediate columnar cells of intestinal metaplasia showing sialomucin and sulphomucin in varying proportion. It was consistently observed that histologically normal mucosa adjacent to malignant tumours of gastrointestinal tract, be it gastric, small intestinal or colic, secrets mucins which differ histochemically and biochemically from normal but resemble those described in foetal gut [2]. Hakkinen et al., have found foetal sulphoglycoprotein which is same histochemically as that of gastric cancer cells. It was suggested that such changes could therefore be further evidence of the reversion to an embryonic state which may be a characteristic of the early stage of carcinogenesis [2].
If we were aware of the normal pattern of mucin secreted by different cells and of the different parts of the stomach, we can easily assess the future types of carcinoma in advance and apply to necessary type of treatment. Any alteration in the mucin pattern from the adjacent areas of any lesion whether it may be ulcerated, precancerous or cancerous lesions, one can easily diagnose the pathology in advance without any other diagnostic modalities.
Conclusion
It was consistently observed that histologically normal mucosa adjacent to malignant tumours of gastrointestinal tract secrets mucins which differ histochemically and biochemically. Such changes could therefore be further evidence of the early stage of carcinogenesis.
Carcinomas of gastrointestinal tract are now classified according to their cellular and morphological features, but it may be possible in future to classify mucin secreting tumours according to composition of their mucins.
M-Magenta, P-Purple, MP-Magenta-purple, B-Blue, BP-Blue-purple, PP-Pink-purple
- ve : Negative staining
± : Weak or variable staining
+ : Slight staining
++ : Moderate staining
+++ : Strong staining
M-Magenta, P-Purple, MP-Magenta-purple, B-Blue, BP-Blue-purple, PP-Pink-purple
- ve : Negative staining
± : Weak or variable staining
+ : Slight staining
++ : Moderate staining
+++ : Strong staining
M-Magenta, P-Purple, MP-Magenta-purple, B-Blue, BP-Blue-purple, PP-Pink-purple
- ve : Negative staining
± : Weak or variable staining
+ : Slight staining
++ : Moderate staining
+++ : Strong staining