Pleural Nocardiosis in an Immunocompetent Patient: A Case Report
Smitha Bagali1, Prakash Mantur2
1 Associate Professor, Department of Microbiology, BLDEU’s Shri. B. M. Patil Medical College, Bijapur, Karnataka, India.
2 Associate Professor, Department of Medicine, BLDEU’s Shri. B. M. Patil Medical College, Bijapur, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr Smitha Bagali, Associate Professor, Department of Microbiology, BLDEU’s Shri. B. M. Patil Medical College, Bijapur-586103, Karnataka, India.
E-mail: drsmithabagali@gmail.com
Nocardiosis is a rare infection that has attracted attention with its increased rate of occurrence in the recent years. In India there is a rare documentation of the pleural involvement in nocardiosis. We report here a case of pleural nocardiosis caused by Nocardia brasiliensis in an immunocompetent patient. This case highlights the importance of considering nocardiosis as a differential diagnosis in patients with pleural lesions.
Nocardia brasiliensis, Pulmonary nocardiosis
Case Report
A 45-year-old male patient presented with complaints of dyspnoea, cough with expectoration and haemoptysis since one month. He also had right sided chest pain, generalized weakness and anorexia. The patient suffered from rheumatic heart disease (RHD) for the past ten years and under penicillin treatment every 21 days. He had no past history of tuberculosis, chronic obstructive pulmonary disease, trauma or any kind of surgery.
On examination, he was thin built. His pulse rate was 90 per minute, blood pressure 110/80 mmHg, respiratory rate 22 per minute and body temperature 98° F. Physical examination revealed bilateral pedal oedema. Examination of the chest revealed diminished breath sounds and dullness to percussion over the right lower lobe region. Examination of the other systems was unremarkable. His haemoglobin was 15.5 gm%, ESR 45 mm/h, total leukocyte count 18,300 /cu. mm with neutrophil 85%. Random blood sugar was 114 g/dl, serum creatinine: 1.1 mg/dl and blood urea: 40 mg/dl. Chest X-ray revealed right sided pleural effusion and cardiomegaly, 2D echocardiography showed RHD with grossly dilated left atrium with severe mitral regurgitation. The ELISA test for human immunodeficiency virus antibodies was negative.
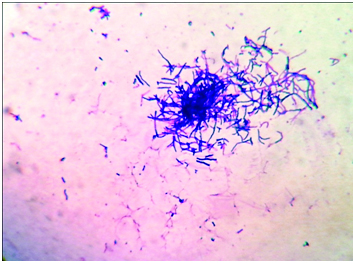
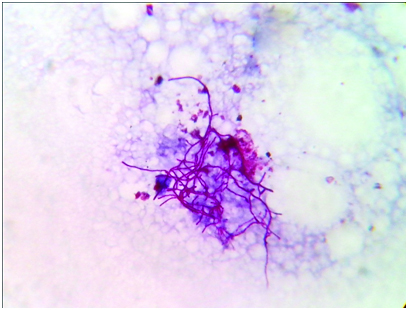
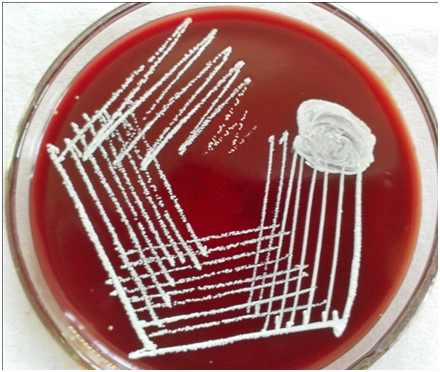
Thoracocentesis was performed after taking written consent from the patient and 600 ml straw coloured pleural fluid was drained. Pleural fluid adenosine deaminase was 210 IU/L. Pleural fluid cytology was negative for malignant cells. Gram stained smear of pleural fluid showed the presence of gram positive branching filamentous bacteria [Table/Fig-1]. Conventional Ziehl Neelsen staining for AFB was negative, however modified Ziehl Neelsen staining using 1% sulphuric acid showed acid fast branching filaments suggestive of Nocardia species [Table/Fig-2]. Blood agar and Sabouraud’s dextrose agar after 48 hours of incubation revealed dry whitish to tan colonies, which on further incubation showed the typical raised, chalky white dry colonies [Table/Fig-3]. Gram stained smear from the culture showed gram positive filamentous bacteria and modified Ziehl-Neelsen staining revealed acid fast filamentous bacteria. Presuming Nocardia, isolate was assigned to traditional grouping based on hydrolysis of casein, tyrosine, xanthine and hypoxanthine. Clearing of tyrosine and hypoxanthine crystals was observed after seven days of incubation indicating the isolate to be Nocardia brasiliensis. Patient was put on empirical antibiotic treatment with piperacillin and tazobactum. However, the patient succumbed before the specific treatment for Nocardia could be initiated.
Photomicrograph of pleural fluid showing gram positive filamentous branching rods. (Gram’s stain, ×1000).
Photomicrograph of pleural fluid showing acid fast branching filamentous bacilli. (Modified Ziehl Neelsen stain, ×1000).
Blood agar plate showing chalky white dry colonies of Nocardia brasiliensis.
Discussion
Nocardiosis is a localized or systemic infection caused by species of the genus Nocardia [1]. Nocardiae are aerobic actinomycetes that are found worldwide in soil, decaying organic plant matter and water [1,2]. More than 50 species of Nocardia have been recognized till now, on the basis of their phenotypic and molecular characters. Among these, around 22 species have been identified as potential pathogens, the most common one being the N. asteroides complex (N. asteroides sensu strictu, N. farcinica, N. nova and N. abscessus). The other medically important species are N. brasiliensis, N. otitidiscaviarum, N. africana, N. brevicatena complex, N. carnea, N. paucivorans, N. pseudobrasiliensis, N. transvalensis and N. veteran [3]. N. asteroides is commonly associated with pulmonary infection and N. brasiliensis with localized skin disease [4]. Most of the Nocardia infections are acquired through inhalation. Organism can also be acquired by direct inoculation of the skin and subcutaneous tissue resulting in primary cutaneous nocardiosis [3].
Pulmonary nocardiosis is an infrequent but serious opportunistic infection that commonly presents as a sub-acute or chronic necrotizing pneumonia, mimicking tuberculosis, lung carcinoma or abscess [1,4]. Pleural involvement in pulmonary nocardiasis is seen in 25% of cases including pleural effusions and empyema [3]. Pleural involvement in nocardiosis is rarely reported from India [5–8]. Nocardia asteroides complex is the most common cause of pleuropulmonary nocardiosis [2,3,9]. Pleural involvement due to Nocardia brasiliensis is rare. Among the Indian studies, two cases of pleural nocardiosis caused by Nocardia brasiliensis have been documented [5,6]. In both the cases aetiological agent was isolated from pleural fluid as in our case.
Nocardiasis occurs mostly in patients that are undergoing corticosteroid therapy or have systemic immunosuppression. The underlying conditions commonly seen in patients with nocardiosis are solid organ transplantation, diabetes, autoimmune diseases, chronic lung disease, chronic granulomatous disease, chronic alcoholism and HIV infection [10,11]. Nocardiosis affects mainly immunocompromised patients but it may also occur in immunocompetent patients. Some previous reports ascertained that 10-20% of patients with nocardiosis had no preexisting illnesss or immunosuppressive therapy [4,10]. The indexed patient was neither immunocompromised nor receiving any corticosteroid therapy. The possible risk factor in our case is presence of rheumatic heart disease as underlying illness predispose the individual to nocardial infection.
Patil et al., have reported a fatal case of pulmonary nocardiosis in an adult male patient with no obvious immunocompromised conditions [12]. But the patient had co-morbid conditions such as diabetes, cardiomyopathy and bronchial asthma. Kontogiorgi M et al., have also reported a case of pulmonary nocardiosis in an elderly immunocompetent individual with Chronic obstructive pulmonary disease [13]. They studied the innate immune responses in the patient and have given evidence for association between defective innate immune responses and occurrence of pulmonary nocardiosis. Another case report by De S et al., described pulmonary nocardiosis mimicking relapse of pulmonary tuberculosis in an immunocompetent young female [14]. The patient was initially diagnosed as pulmonary tuberculosis for which she received antitubercular treatment for six months. After completion of therapy the patient presented with cavitary lesion in the left upper zone and later diagnosis of pulmonary nocardiosis was ascertained.
Trimethoprim-sulfamethoxazole remains the drug of choice for the treatment of nocardiosis. At times, sulfa drug cannot be used for treatment due to allergy, intolerance, toxicity or treatment failure with sulfa drug. To choose an alternative drug, the infecting Nocardia species and susceptibility pattern should be taken into consideration. Non sulfa drugs found effective against Nocardia species are amikacin, minocycline, imipenem, meropenem, ceftriaxone, cefotaxime, erythromycin, moxifloxacin, levofloxacin, linezolid, tigecycline, and ticarcilin/clavulanic acid. In view of the propensity of relapsing disease treatment is required for a prolonged period, which may vary from 6-12 months [15].
Conclusion
This particular case emphasizes the need for high index of clinical suspicion of pulmonary nocardiosis. Nocardiosis should be considered even in a patient without any risk factor, particularly if they are not responding to treatment for a more common infection.
[1]. Lerner PI, NocardiosisClin Infect Dis 1996 22:891-905. [Google Scholar]
[2]. Saubolle MA, Sussland D, Nocardiosis: Review of clinical and laboratory experienceJ Clin Microbiol 2003 41:4497-501. [Google Scholar]
[3]. Vohra P, Sharma M, Yadav A, Chaudhary U, Nocardiosis: A review of clinico-microbiological featuresInt J LifeSc Bt Pharm Res 2013 2:20-28. [Google Scholar]
[4]. Menendez R, Cordero PJ, Santos M, Gobernado M, Marco V, Pulmonary infection with Nocardia species: a report of 10 cases and reviewEur Respir J 1997 10:1542-46. [Google Scholar]
[5]. Gowrinath K, Rao PS, Mohapatra AK, Prakash PY, Pleural nocardiosisIndian J Chest Dis Allied Sci 2009 51:169-71. [Google Scholar]
[6]. Shiva prakash MR, Rao P, Mandal J, Biswal M, Gupta S, Ray P, Nocardiosis in a tertiary care hospital in North India and review of patients reported from IndiaMycopathologia 2007 163:267-74. [Google Scholar]
[7]. Gowrinath K, Das S, Ranjitham M, Shekar U, Thanaskaran V, Nocardial hydropneumothoraxIndian J Chest Dis Allied Sci 2004 46:51-53. [Google Scholar]
[8]. Gupta E, Dhawan B, Thabah MM, Das BK, Sood S, Kapil A, Nocardia pyopneumothorax in an immunocompetent patientIndian J Med Res 2006 124:363-64. [Google Scholar]
[9]. Hui CH, Au VW, Rowland K, Slavotinek JP, Gordan DL, Pulmonary nocardiosis re-visited: Experience of 35 patients at diagnosisRespir Med 2003 97(6):709-17. [Google Scholar]
[10]. Yang M, Xu M, Wei W, Gao H, Zhang X, Zhao H, Clinical findings of 40 patients with nocardiosis: A retrospective analysis in a tertiary hospitalExp Ther Med 2014 8:25-30. [Google Scholar]
[11]. Chen J, Zhou H, Xu P, Zhang P, Ma S, Zhou J, Clinical and Radiographic Characteristics of Pulmonary Nocardiosis: Clues to Earlier DiagnosisPLoS ONE 2014 9(3):e90724 [Google Scholar]
[12]. Patil M C S, Varghese J, Rajagopalan N, A fatal case of pulmonary nocardiosisBMJ Case Reports 2012 2012:bcr0920114875-bcr0920114875. [Google Scholar]
[13]. Kontogiorgi M, Opsimoulis P, Kopterides P, Savva A, Kalodimou V, Belesiotou E, Pulmonary nocardiosis in an immunocompetent patient with COPD: The role of defective innate responseHeart & Lung: The Journal of Acute and Critical Care 2013 42(4):247-50. [Google Scholar]
[14]. De S, Desikan P, Pulmonary nocardiosis mimicking relapse of tuberculosisBMJ Case Reports 2009 2009:bcr0620080233-bcr0620080233. [Google Scholar]
[15]. Wilson J, Nocardiosis: Updates and Clinical OverviewMayo Clinic Proceedings 2012 87(4):403-07. [Google Scholar]